Methods for treating patients with hematologic malignancies
A technology of malignant blood diseases and leukemia cells, which is applied in the field of treating patients with malignant blood diseases, and can solve the problems of poor survival and prognosis of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1: Synthesis of 1-{3-fluoro-4-[7-(5-methyl-1H-imidazol-2-yl)-1-oxo-2,3-dihydro-1H-isoindole- 4-yl)-phenyl)-3-(2,4,6-trifluoro-phenyl)-urea (compound 7)
[0182]
[0183] 2,4,6-Trifluorobenzoic acid (0.08g, 0.45mmol) was dispersed in ether (5.7mL), and phosphorus pentachloride (PCl 5 , 0.11g, 0.52mmol), and then stirred for 1 hour. After the reaction was completed, the organic solvent was concentrated under reduced pressure below room temperature, and then the reaction solution was diluted by adding acetone (3.8 mL). Subsequently, sodium azide (NaN) dissolved in water (0.28 mL) at 0°C 3 , 0.035g, 0.545mmol) was slowly added dropwise to the reaction solution. After stirring for 2 hours at room temperature, the 2,4,6-trifluorobenzoyl azide thus formed was diluted with ethyl acetate, followed by washing with water. The organic layer was dried over anhydrous magnesium sulfate, dispersed in THF (2mL), and added containing 4-(4-amino-2-fluorophenyl)-7-(5-methyl-1H-imida...
Embodiment 2
[0184] Example 2: Binding constant of compound 7 to wild-type and mutant FLT3 kinase
[0185] The measurement of the kinase activity is called the binding constant or K d value. The scheme used to obtain these values is described accordingly. For most assays, a kinase-tagged T7 phage strain was prepared in an E. coli host derived from the BL21 strain. Escherichia coli was grown to log phase and infected with T7 phage and incubated at 32°C with shaking until dissolved. The lysate is centrifuged and filtered to remove cell debris. The remaining kinases are produced in HEK-293 cells and subsequently tagged with DNA for qPCR detection. The streptavidin-coated magnetic beads were treated with a biotinylated small molecule ligand at room temperature for 30 minutes to generate an affinity resin for kinase assay. Block the ligandized beads with excess biotin and wash with blocking buffer (SeaBlock (Pierce), 1% BSA, 0.05% Tween 20, 1 mM DTT) to remove unbound ligands and allow non-s...
Embodiment 3
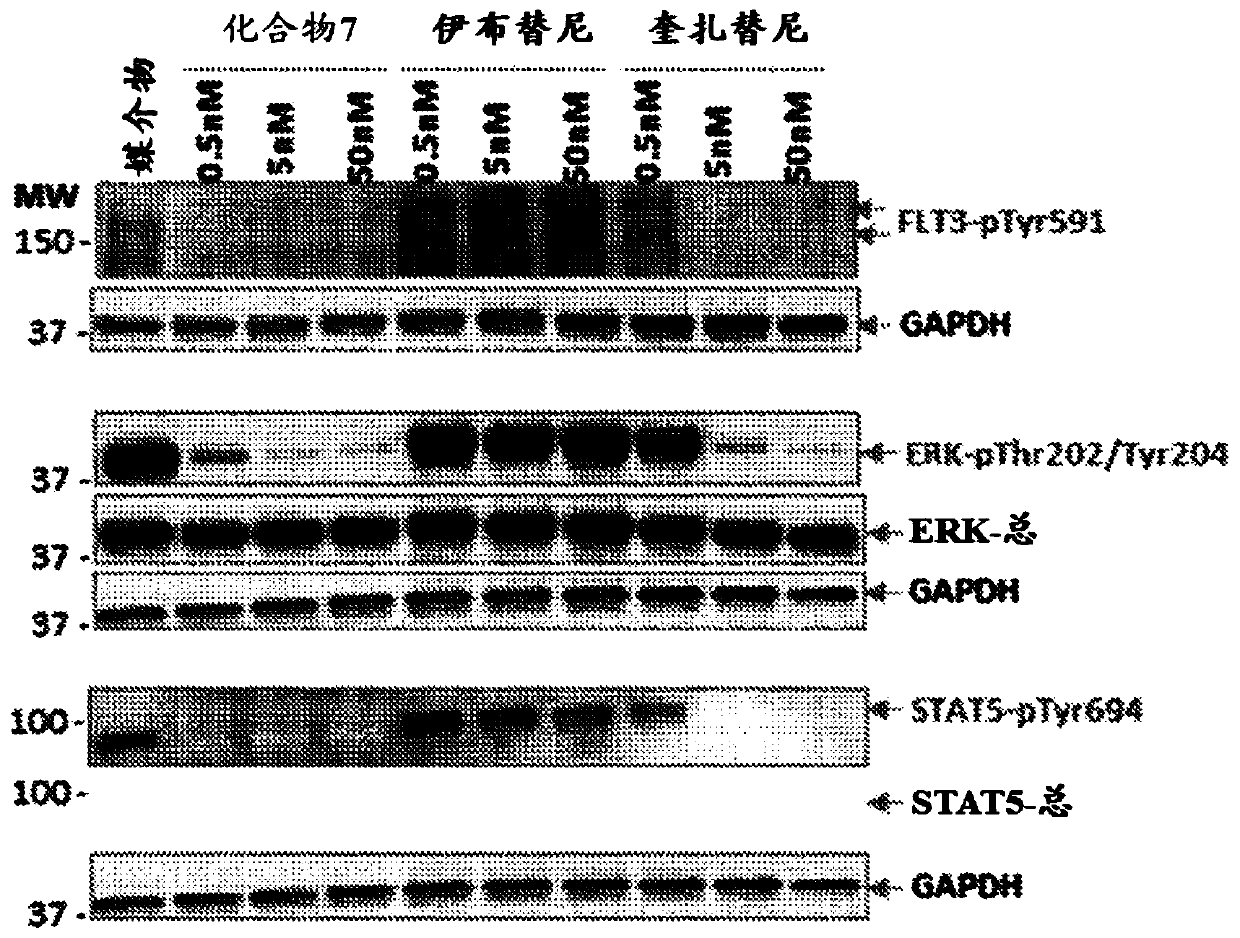
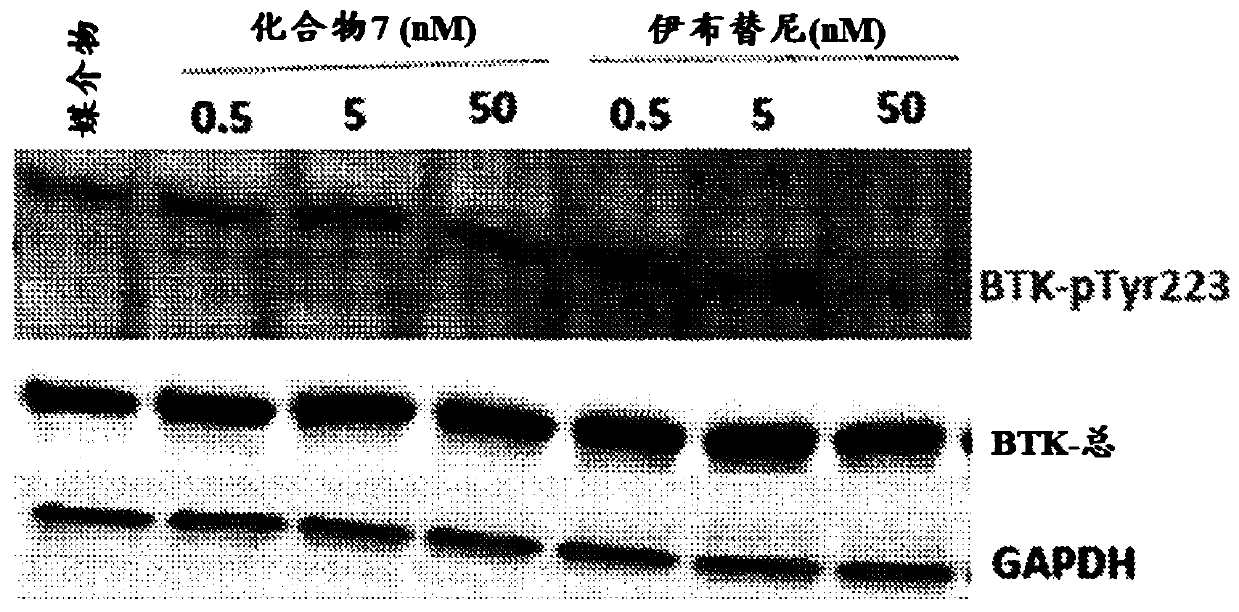
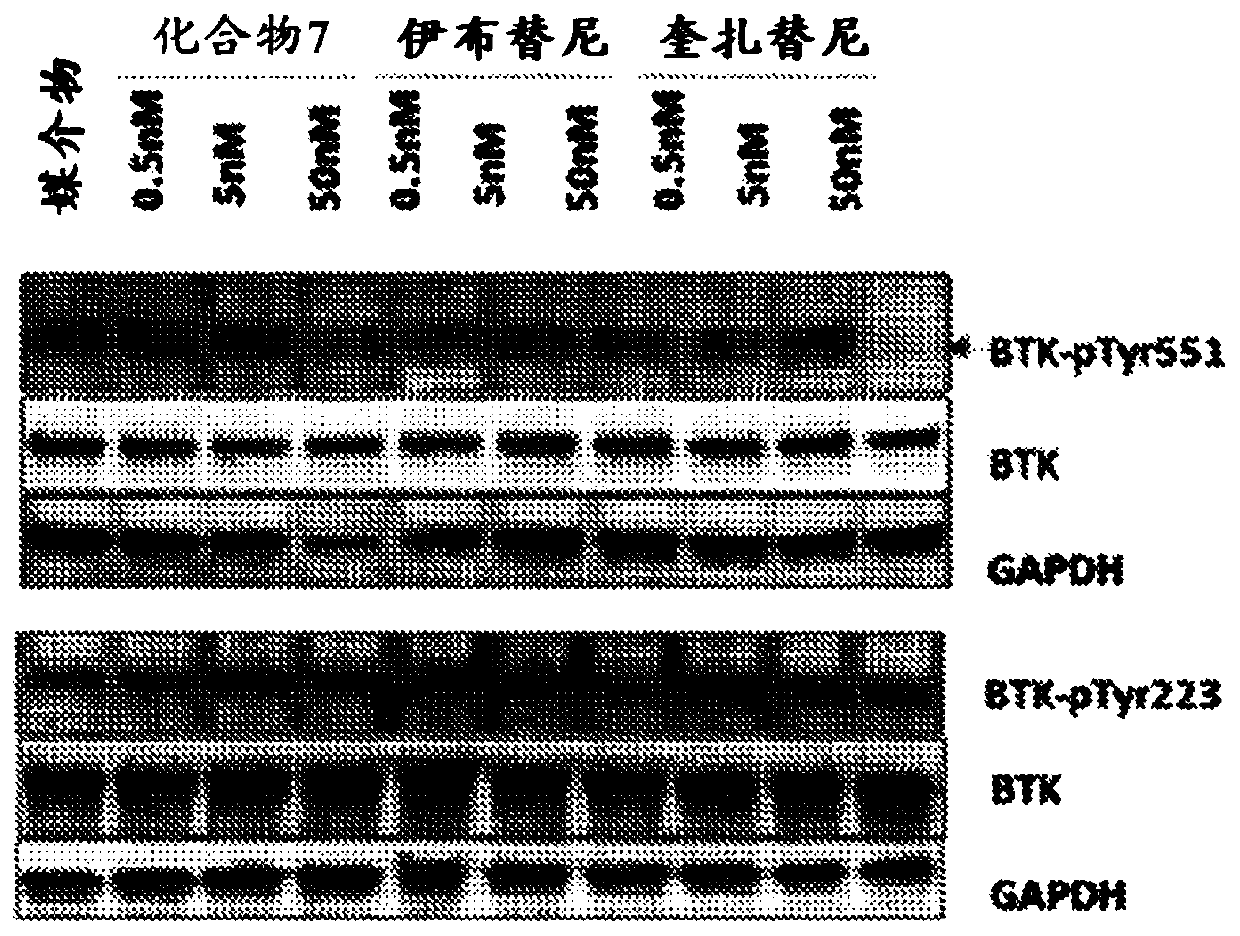
[0190] Example 3: Western blot analysis of compound 7 in the case of MV4-11 cells
[0191] figure 1 Shows the results of Western blot analysis. Without being bound by any theory, this proves that compound 7 inhibits the FLT3 pathway in MV4-11 cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com