Fusion gene vector construction and expression as well as uses
A carrier and gene technology, which is applied in the application field of preparing tumor biotherapeutic drugs, can solve the problems of enhancing antigen presentation due to stimulation, and cannot ensure that both genes are transfected into the same cell, so as to enhance immune response and expand Effect of anti-tumor range and reduction of medical expenses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Materials
[0045] 1.1.1 Strains and plasmids E. coli competent cells DH5α; eukaryotic expression plasmids PCDNA3.1(+) / hTERT and PCDNA3.1(+).
[0046] 1.1.2 Reagents Restriction enzymes Hind III, Nhe I, EcoR I, Not I, T4 DNA ligase, Taq DNA polymerase, dNTPs, reverse transcription kit, plasmid extraction kit, etc. were purchased from Promega, USA; IL-18 and TERT antibodies and γ-interferon ELISA kits were purchased from R&D Company, total RNA extraction kit was from Invitrogene Company; apoptosis detection kit was purchased from Bender Company; MTX was purchased from Sigma Company; yeast extract, pancreatic Bacterial culture reagents such as peptone were produced by Oxford, UK; agarose, RNase, and gel recovery kits were produced by Sangon; calcium chloride, SDS, lymphocyte separation solution, and sodium acetate were domestic reagents.
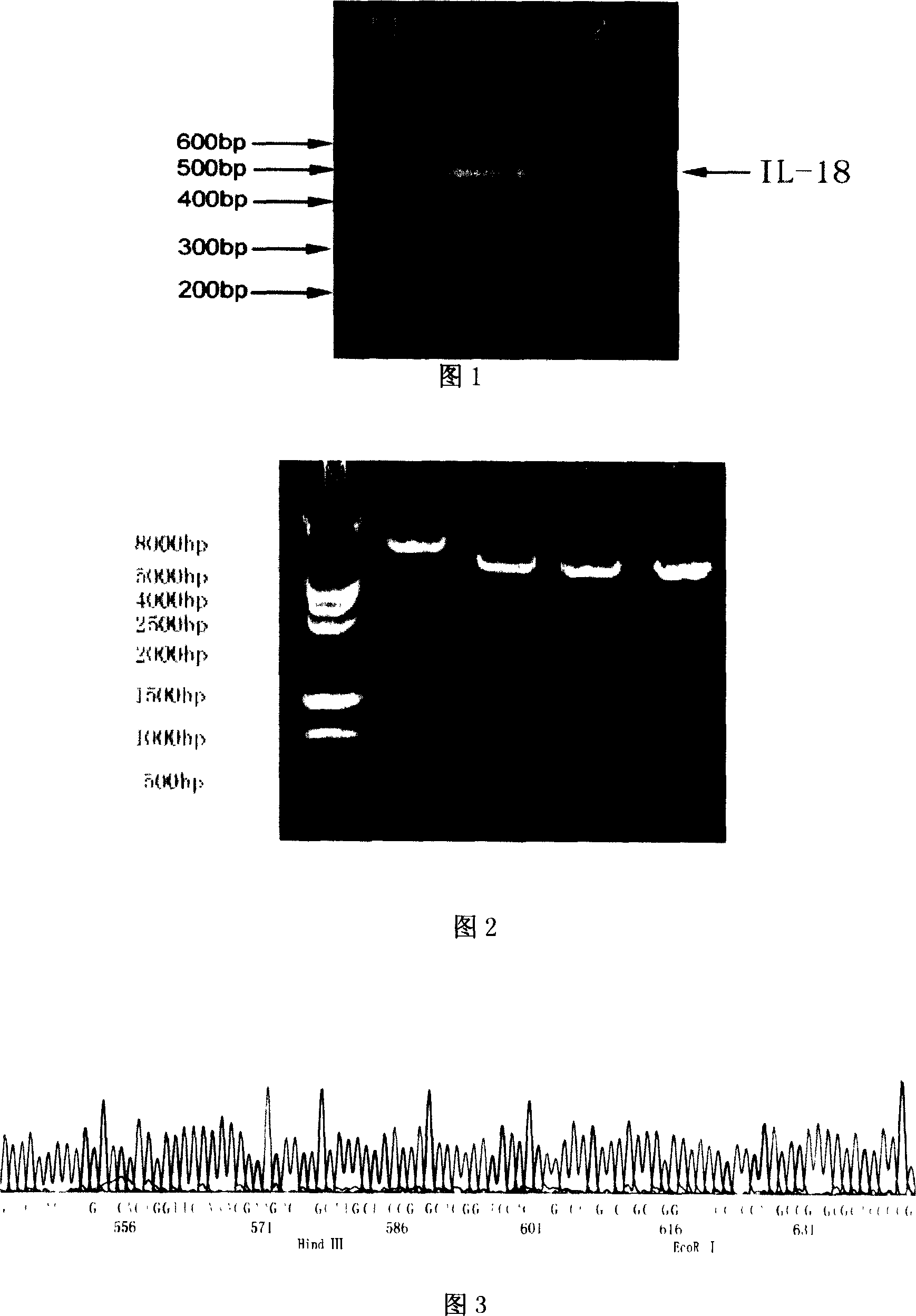
[0047] 1.1.3PCR primers The primers for IL-18 gene cloning were designed with reference to the sequence registered in Genebank, de...
Embodiment 2
[0068] Example 2 Detection of Biological Function of Human Telomerase Reverse Transcriptase (hTERT) / Human Interleukin 18 (hIL18) Fusion Gene
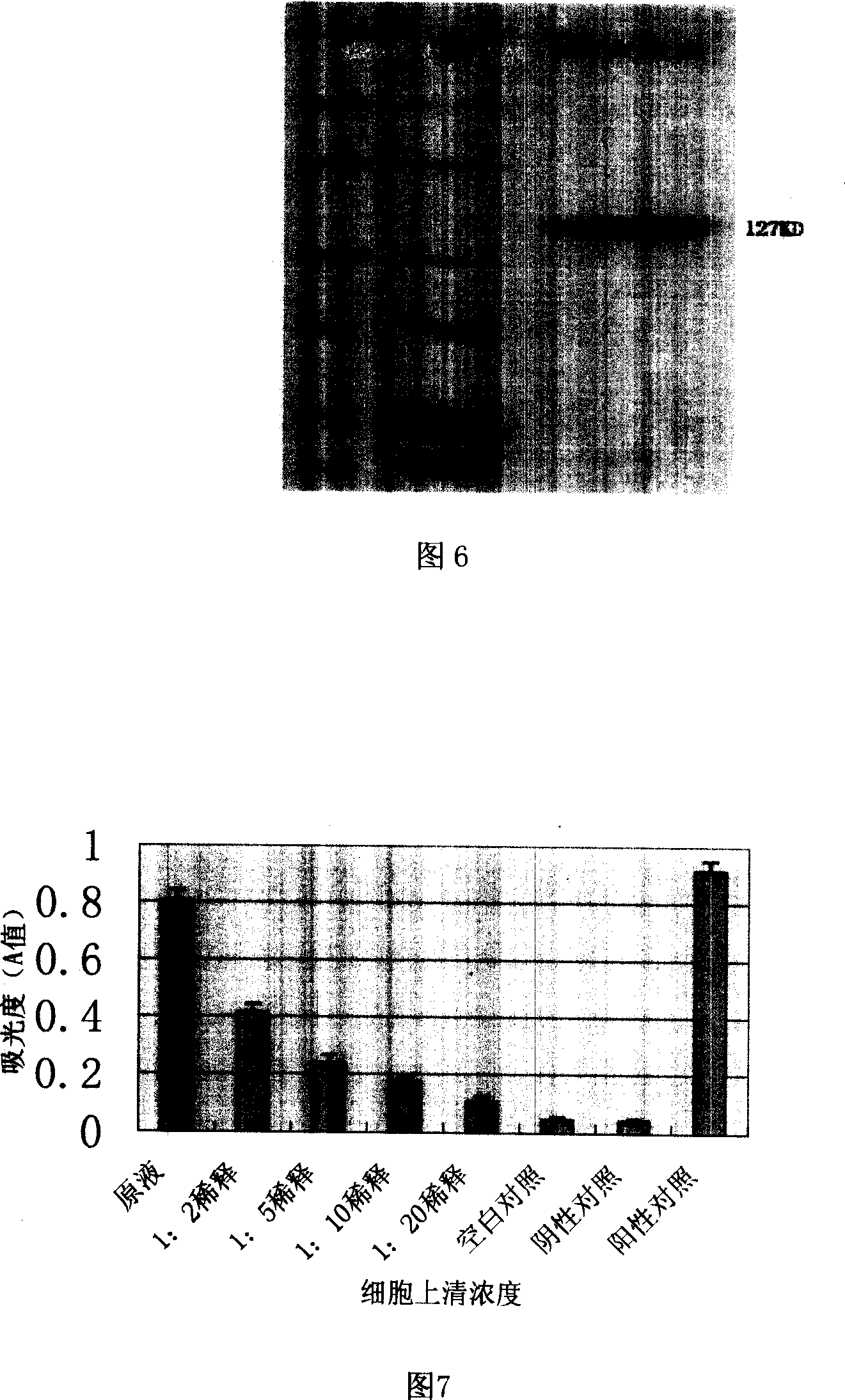
[0069] 1. Detection of biological function of fusion gene stimulating KG-1 cells to secrete γ-interferon
[0070] Detection of γ-interferon content in cell culture supernatant: take out 0.1ml of culture supernatant of 3T3 cells transfected with fusion gene, from 1×10 6 Take 0.1ml of the KG-1 cells per ml to a 96-well plate, and use the original solution, 1:2 dilution, 1:5 dilution, 1:10 dilution, 1:20 dilution, normal 3T3 cell culture supernatant (negative control ), and standard IL-18 stimulation solution (positive control) and blank control (PBS). Three replicate wells were set up for each concentration. Quantitative enzyme-linked immunosorbent technology (ELISA method) was used, and the operation steps were carried out according to the instructions of the kit. Absorbance (A value) was measured at a wavelength of 490 nm on a microp...
Embodiment 3
[0074] 1. Electrotransfection of dendritic cells with fusion gene
[0075] (1) Take 2×10 6 cells, washed twice with serum-free and antibiotic-free medium, suspended in 400 μl of hypo-osmolar
[0076] buffer (product of Eppendorf Company) electroporation buffer.
[0077] (2) Add 10 micrograms of sterile pyrogen-free fusion gene plasmid to the above cell suspension, mix gently, and transfer to a 400 microliter electroporation cuvette with 2mm spacing and 400ul volume.
[0078] (3) Place the electrospinning cup in the electroporator (Multiporator Eppendorf), and 300V / 20us electric current shocks twice with an interval of 1 minute.
[0079] (4) Aspirate the cells after electroporation, wash the cells twice with 10 ml serum-containing medium (800 rpm×5 min); and culture in RPMI1640 medium with 15% FCS.
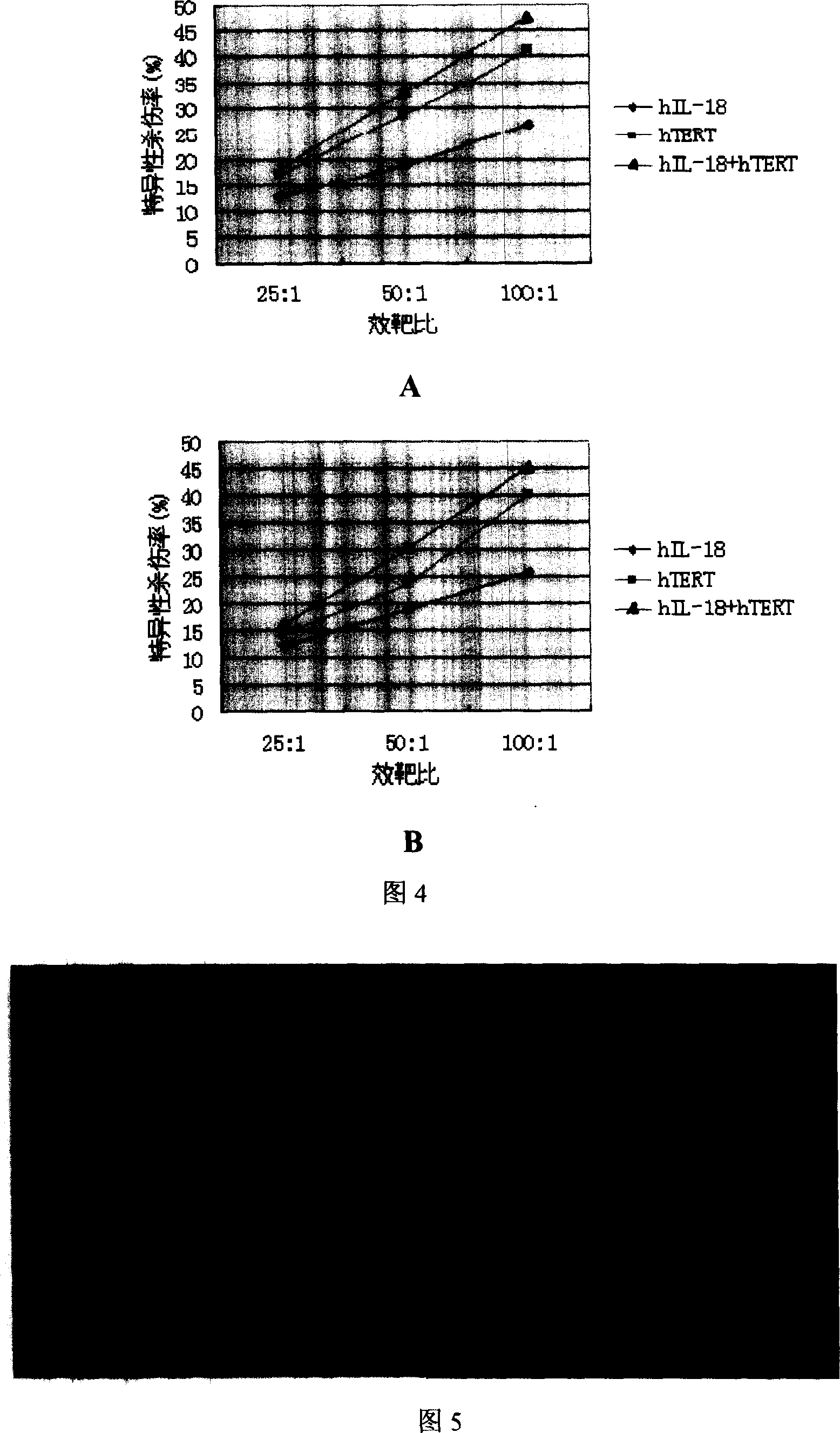
[0080] 2. Induction of cytotoxic T lymphocytes (CTL) in vitro and detection of killing activity
[0081] T lymphocytes from normal human peripheral blood were isolated, and co-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com