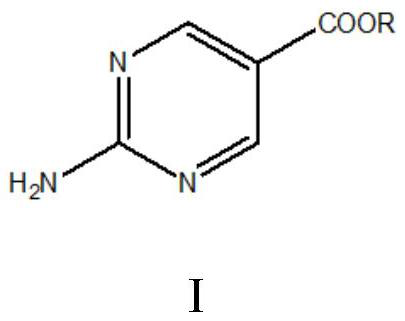
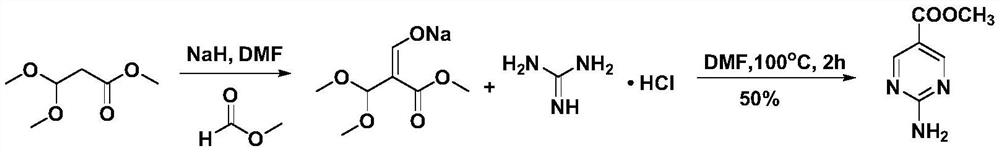
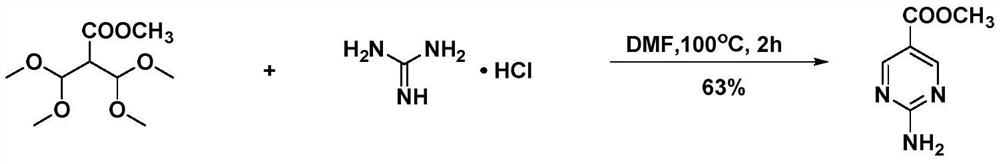
A kind of convenient preparation method of 2-aminopyrimidine-5-carboxylate
A technology of aminopyrimidine and formate, applied in the field of medicinal chemistry, can solve the problems of difficulty in obtaining, high price, no industrialization value, etc., and achieves the effects of simple operation, low cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of ethyl 2-aminopyrimidine-5-carboxylate
[0043] In the 500 milliliters of four-neck flasks that are connected with stirring, thermometer, reflux condenser and 20wt% aqueous sodium hydroxide tail gas absorption device, add 200 grams of carbon tetrachloride, 22.8 grams (0.2 moles) of ethyl methacrylate, 0.5 g of benzoyl peroxide, under stirring, intermittently feed chlorine gas at 65-70°C, 29 g of chlorine gas in total for 2 hours, stir and react at 65-70°C for 3 hours, then nitrogen bubbles for 1 hour to replace residual chlorine and hydrogen chloride. Cool to room temperature, add 0.15 g of N,N-dimethylformamide to eliminate benzoyl peroxide; intermittently feed chlorine gas, control the temperature not to exceed 40°C, feed 15 g of chlorine gas for 2 hours, stir at 40-45°C React for 5 hours. Recover carbon tetrachloride by distillation, add 300 grams of 75wt% ethanol, 30 grams of guanidine nitrate, 80 grams of potassium carbonate, stir and r...
Embodiment 2
[0046] Embodiment 2: Preparation of 2-aminopyrimidine-5-methyl carboxylate
[0047]In the 500 milliliters of four-necked flasks that are connected with stirring, thermometer, reflux condenser and 20wt% aqueous sodium hydroxide tail gas absorption device, add 200 gram carbon tetrachlorides, 20.0 gram (0.2 moles) methyl methacrylate, 0.5 g of benzoyl peroxide, under stirring, intermittently feed chlorine gas at 65-70°C, 29 g of chlorine gas in total for 2 hours, stir and react at 65-70°C for 3 hours, then nitrogen bubbles for 1 hour to replace residual chlorine and hydrogen chloride. Cool to room temperature, add 0.15 g of N,N-dimethylformamide to eliminate benzoyl peroxide; intermittently feed chlorine gas, control the temperature not to exceed 40°C, feed 15 g of chlorine gas for 2 hours, stir at 40-45°C React for 5 hours. Recover carbon tetrachloride by distillation, add 300 grams of methanol, 45 grams of sodium hydroxide, 30 grams of guanidine nitrate, stir and react at 60-6...
Embodiment 3
[0048] Embodiment 3: Preparation of ethyl 2-aminopyrimidine-5-carboxylate
[0049] In the 500 milliliters of four-neck flasks that are connected with stirring, thermometer, reflux condenser and 20wt% aqueous sodium hydroxide tail gas absorption device, add 250 gram carbon tetrachlorides, 22.8 gram (0.2 mole) ethyl methacrylates, 0.5 grams of benzoyl peroxide, 36.0 grams of N-bromosuccinimide, stirred and reacted at 65-70 ° C for 5 hours, cooled to 10-15 ° C, filtered, and the filtrate was transferred to a device with stirring, thermometer, reflux condensation In a four-neck flask with a constant pressure low liquid funnel, add 0.15 g of N,N-dimethylformamide to eliminate benzoyl peroxide; add 32.0 g of bromine and 50 g of tetrachloromethane dropwise at 50-60°C Carbonization solution, after about 1 hour drop, stirred and reacted at 55-60°C for 3 hours. Recover carbon tetrachloride by distillation, add 300 grams of 75wt% ethanol, 30 grams of guanidine nitrate, 80 grams of potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com