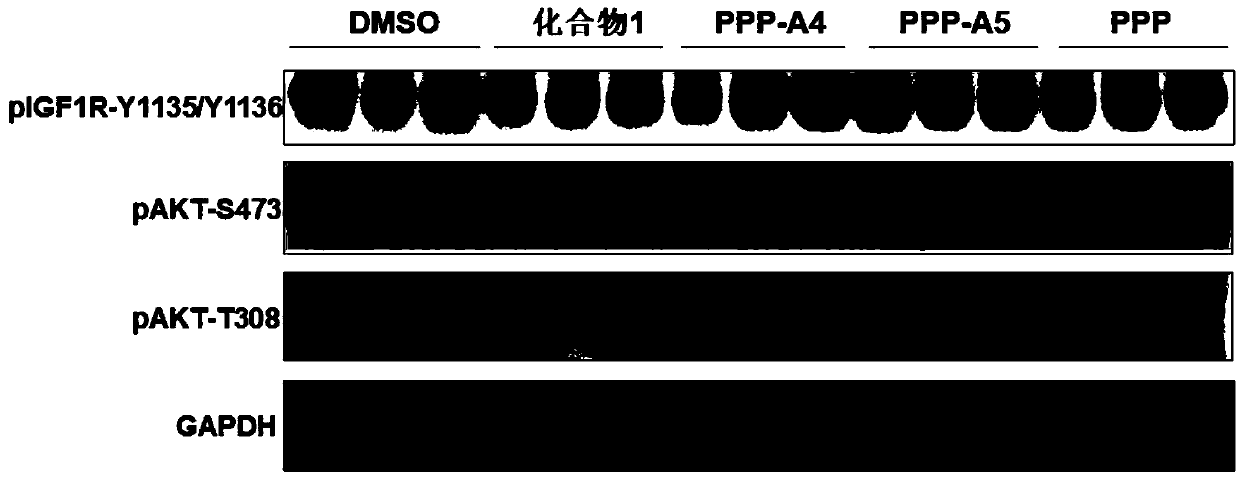
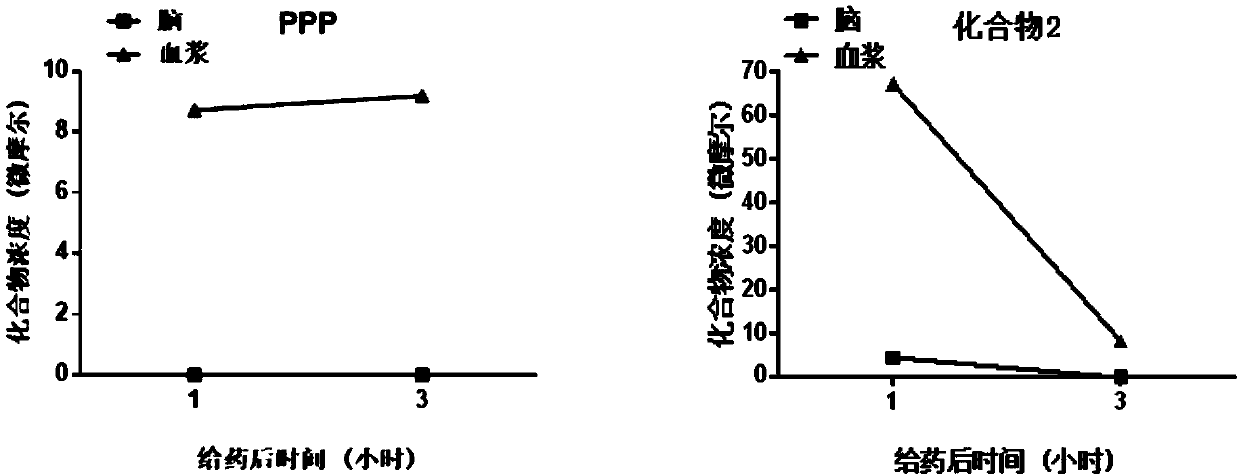
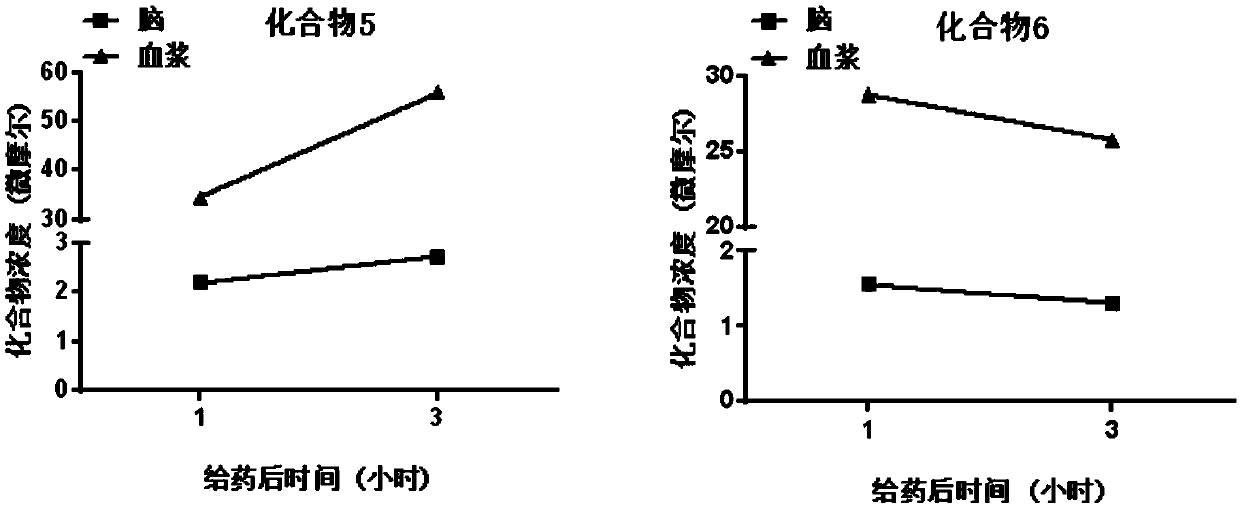
Insulin-like growth factor-1 receptor tyrosine kinase inhibitor and uses thereof
A use, halogen technology, applied in the field of medicinal chemistry, can solve problems such as poor BBB permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Compound 1: Rel-(5R,5aS,8aR,9R)-2,2-difluoro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5, 8,8a,9-Tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one
[0064]
[0065] Synthesis of Intermediate 1.1: 4-vinyldihydrofuran-2(3H)-one
[0066]
[0067] 2-Butene-1,4-diol (206.4 g, 2.34 mol, 1.0 e.q.) was dissolved in triethyl orthoacetate (546.7 g, 3.4 mol, 1.4 e.q.) with stirring, and catalytic hydroquinone (54.00 g , 0.49mol, 0.2e.q.), and the resulting mixture was heated to 120°C. The ethanol formed in situ is continuously distilled off until no more ethanol is produced. The temperature was raised to 150 °C and the reaction mixture was stirred for an additional 48 hours. Intermediate 1.1 was collected by vacuum distillation (70-75°C, 2-3 mmHg) as a colorless oil (170.0 g, yield 65%). 1 HNMR (400MHz, CDCl 3 )δ (ppm) 2.36 (dd, J = 17.4Hz, 8.7Hz, 1H), 2.69 (dd, J = 17.7Hz, 8.4Hz, 1H), 3.19-3.29 (m, 1H), 4.01-4.14 (m, 1H), 4.43-4.47(m, 1H), 5.17-5.23(m, 2H)...
Embodiment 2
[0087] Compound 2: Rel-(5R,5aR,8aS,9R)-2,2-difluoro-8-oxo-9-(3,4,5-trimethoxyphenyl)-5, 5a,6,8,8a,9-Hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl Acetate
[0088]
[0089] Compound 1 (1.15 g, 2.55 mmol, 1.0 e.q.) was dissolved in DCM (30 mL) with stirring, Et 3 N (770mg, 7.6mmol, 3.0e.q.), 4-dimethylaminopyridine (310mg, 2.55mmol, 1.0e.q.), acetyl chloride (400mg, 5.1mmol, 2.0e.q.) was added at ice-water bath temperature, and the resulting mixture was Stir at ambient temperature for 12 hours. The reaction mixture was quenched with saturated aqueous ammonium chloride, and the aqueous phase was extracted with additional DCM. The combined organic phases were washed with brine, dried over magnesium sulfate, filtered and concentrated. The obtained crude product was recrystallized from PE / EtOAc to afford compound 2 (810 mg, 65% yield) as a white solid. 1 HNMR (400MHz, CDCl3) δ (ppm) 7.02 (s, 1H), 6.76 (s, 1H), 6.41 (s, 2H), 5.81 (d, J = 6.3Hz, 1H), 4.48-4.34 (m, ...
Embodiment 3
[0091] Compound 3: Rel-(5R,5aS,8aR,9S)-2,2,9-trifluoro-5-(3,4,5-trimethoxyphenyl)-5,8,8a, 9-Tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one
[0092]
[0093] Compound 1 (700 mg, 1.60 mmol, 1.0 e.q.) was dissolved in DCM (20 mL) with stirring, diethylaminosulfur trifluoride (520 mg, 3.20 mmol, 2.0 e.q.) was added dropwise, and the resulting mixture was stirred at room temperature 12 hours. The reaction mixture was quenched with saturated aqueous sodium bicarbonate and stirred for an additional 30 minutes. The organic phase was separated and the aqueous phase was extracted with additional DCM. The combined organic phases were washed with brine, dried over magnesium sulfate, filtered and concentrated. Purification by column chromatography (200-300 mesh silica gel, DCM / MeOH=100 / 1-50 / 1) gave compound 3 (130 mg, 18% yield) as a white solid. 1 HNMR (400MHz, CDCl 3 )δ(ppm)7.27(s,1H),6.66(s,1H),6.44(s,2H),5.49-5.34(m,1H),4.60-4.47(m,2H),4.20(d,J= 5.0Hz, 1H), 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com