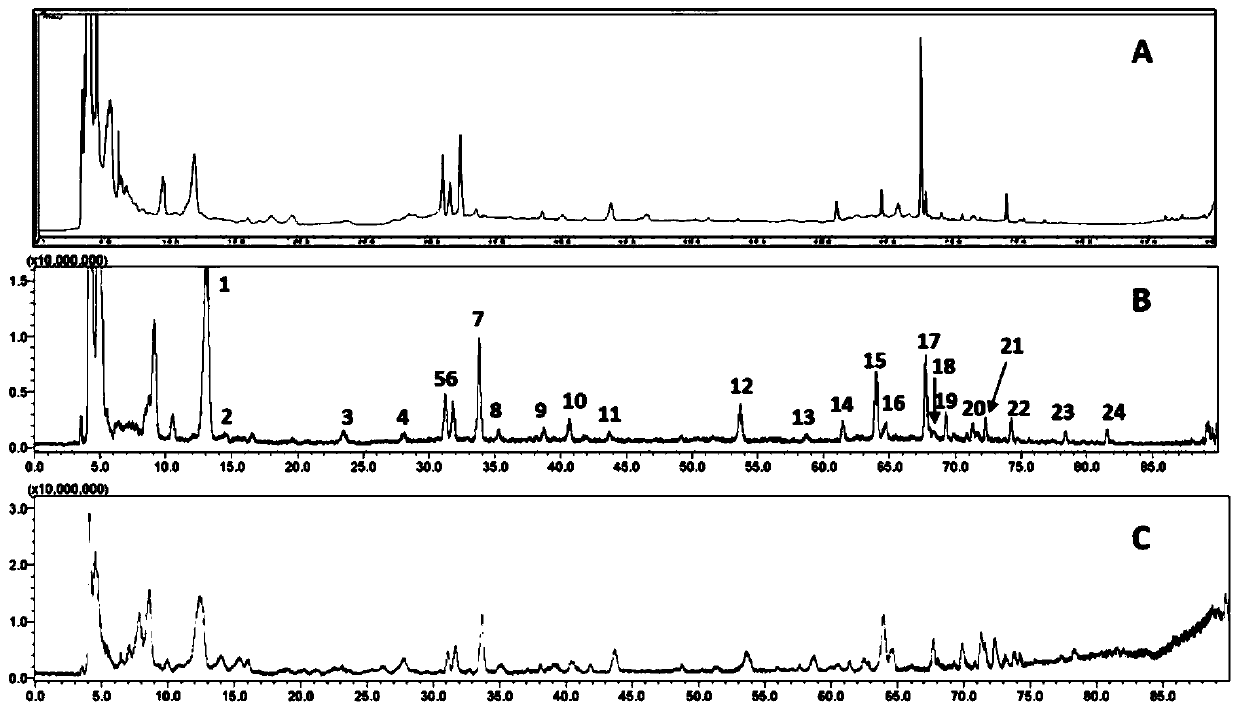
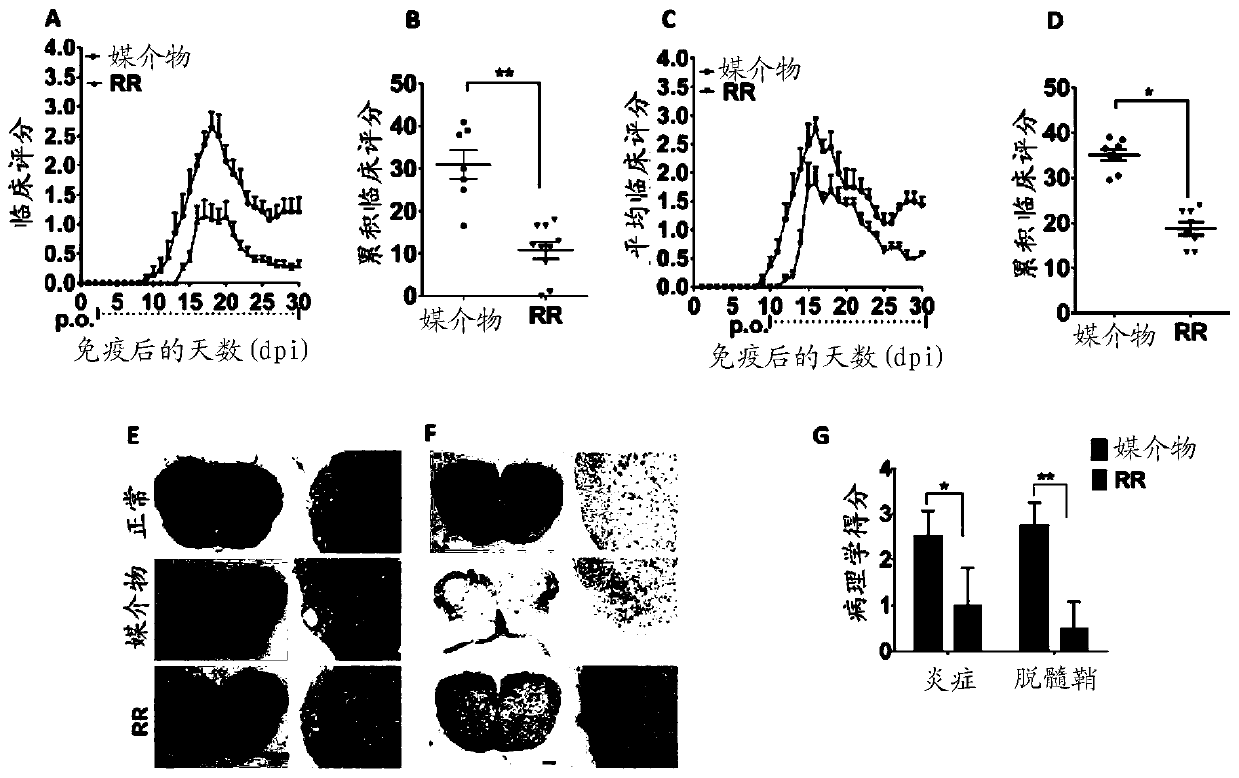
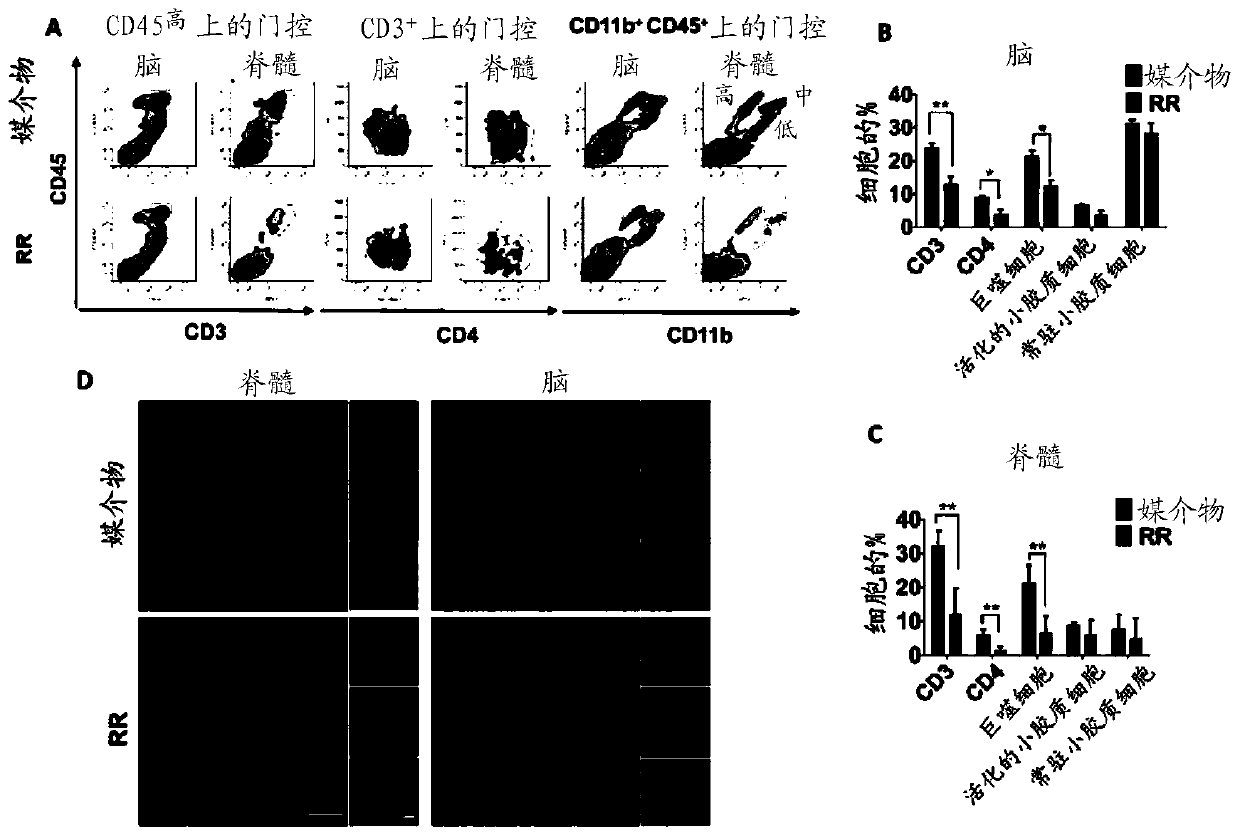
Extracts from Rehmannia glutinosa as a therapeutic agent for multiple sclerosis
A technology of multiple sclerosis and extracts, which can be used in drug combinations, plant raw materials, allergic diseases, etc., and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Reagent
[0079] Rehmannia glutinosa was purchased from KANG MEI Pharmaceutical Co., Ltd (Guangdong, China). Mouse myelin oligodendrocyte glycoprotein (35-55) peptide (MOG) with a purity of more than 96% (wt / wt) 35-55 , MEVGWYRSPFSRVVHLYRNGK) were purchased from Chinese Peptide Company (Zhejiang, China), Freund’s incomplete adjuvant was from Sigma-Aldrich (St.Louis, MO, USA), Mycobacterium tuberculosis H37RA was from BD Biosciences (Difco, BD), and pertussis toxin From List Biological Laboratories (CA, USA). Percoll gradients were purchased from GE Healthcare Life Sciences (Pittsburgh, PA, USA). Cell surface staining antibodies were obtained from eBioscience (San Diego, CA, USA), including CD45-PE (30-F11), CD3e-FITC (145-2C11), CD4-Pacific Blue (RM4-5), and CD11b-APC (M1 / 70). Primary antibodies to 3-NT and iNOS were obtained from Abcam (Cambridge, UK); Bax, p-p65 Ser536 ,p65,p-IKKα / β Ser176 / 180 , IKKβ, p-IκBα Ser32 , IκBα and GAPDH from Cell signaling Technology...
Embodiment 2
[0137] method
[0138] animal
[0139] Female C57BL / 6N mice (10-14 weeks old) were obtained from the Laboratory Animal Unit of the University of Hong Kong. All animal care and experimental procedures were approved by the University Committee on the Use of Live Animals in Teaching and Research (CULATR). Animals were housed in a pathogen-free environment with a 12-hour dark / light cycle.
[0140] Active EAE induction and drug therapy
[0141] Female C57BL / 6N mice were immunized to actively induce EAE as previously described by the inventors (Li, W., et al., 2018). Briefly, with 200 μg MOG in complete Freund's adjuvant (5 mg / ml, Sigma-Aldrich) 35-55 Inject mice subcutaneously. Pertussis toxin (200 ng, List Biological Laboratories) was injected intravenously on days 0 and 2 (dpi.) after immunization. Daily monitoring of immunized mice was assessed with body weight measurements and clinical scores. To assess clinical severity, EAE symptoms were scored as follows: 0, no clinic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com