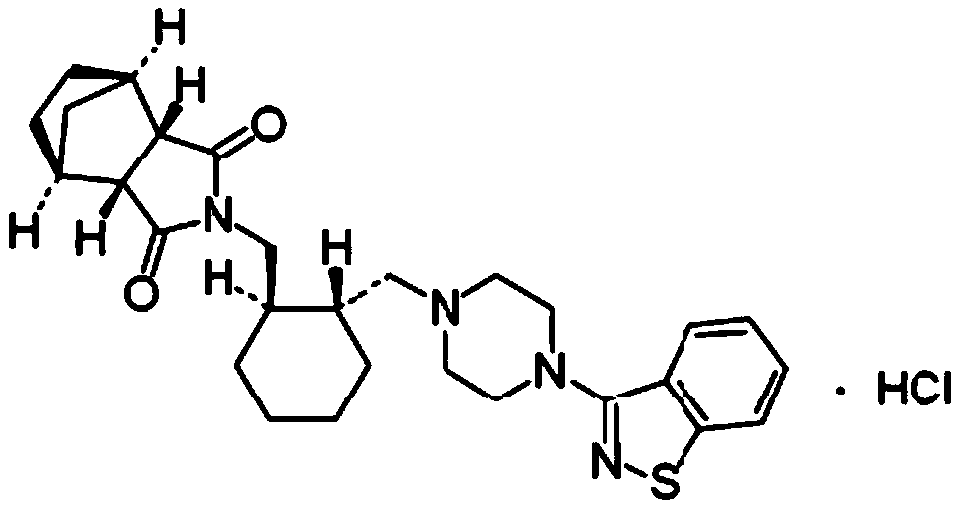
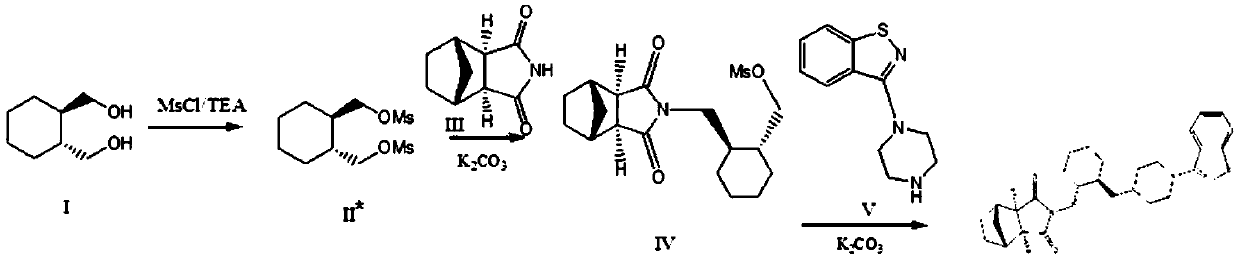
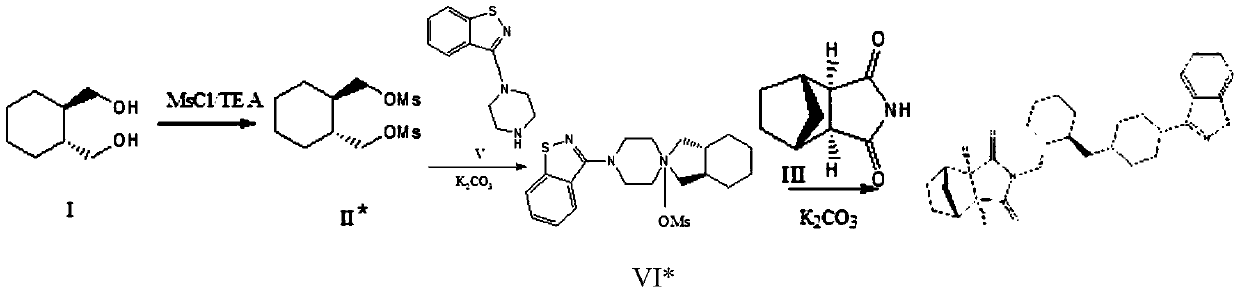
Method for preparing lurasidone and salt thereof
A technology of lurasidone hydrochloride and intermediate, which is applied in the field of medicine and can solve problems such as difficulty in controlling impurities in the intermediate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Embodiment 1: Preparation of lurasidone hydrochloride
[0116] Step 1, preparation of intermediate I
[0117] (R,R)-1,2-bis(methylsulfonyloxymethyl)cyclohexane (which can be referred to as raw material 1 in the present invention) 300.4g (1mol), 3-(1-piperazinyl )-1,2-benzisothiazole hydrochloride (it can be referred to as raw material 2 in the present invention) 1mol, anhydrous potassium carbonate 2mol, acetonitrile (its amount is 15 times of raw material 1 weight) add in the reactor , Stir the reaction at 70°C until TLC detection (acetone / ethyl acetate=1:9) until the spot of the starting material (raw material 1) disappears; cool to room temperature, filter, and concentrate the filtrate to 1 / 3 volume by distillation under reduced pressure, then add Ethanol (its amount is 5 times of raw material 1 weight), stirred for 1.5 hours, and the solvent was evaporated; Added toluene (its amount was 6 times of raw material 1 weight), stirred at 70 ° C for 1.5 hours, evaporated...
Embodiment 2
[0122] Embodiment 2: Preparation of lurasidone hydrochloride
[0123] Step 1, preparation of intermediate I
[0124] (R,R)-1,2-bis(methylsulfonyloxymethyl)cyclohexane 300.4g (1mol), 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride Add 1.1 mol of salt, 1.8 mol of anhydrous potassium carbonate, and acetonitrile (the amount is 12 times the weight of raw material 1) into the reaction kettle, stir and react at 80°C until TLC detection (acetone / ethyl acetate=1:9) The spots of the starting material (raw material 1) disappeared; cooled to room temperature, filtered, and the filtrate was concentrated to 1 / 3 volume by distillation under reduced pressure, then added ethanol (its amount was 4 times the weight of raw material 1), stirred for 2 hours, and evaporated the solvent; Toluene (the amount is 5 times the weight of raw material 1), stirred at 80°C for 1 hour, evaporated to remove the solvent to concentrate the material to 2 / 3 volume, cooled to 2-8°C, filtered, and the filter c...
Embodiment 3
[0129] Embodiment 3: Preparation of lurasidone hydrochloride
[0130] Step 1, preparation of intermediate I
[0131] (R,R)-1,2-bis(methylsulfonyloxymethyl)cyclohexane 300.4g (1mol), 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride Add 0.9 mol of salt, 2.2 mol of anhydrous potassium carbonate, and acetonitrile (the amount is 18 times the weight of raw material 1) into the reaction kettle, stir and react at 60°C until TLC detection (acetone / ethyl acetate=1:9) The spots on the starting material (raw material 1) disappeared; cooled to room temperature, filtered, and the filtrate was concentrated to 1 / 3 volume by distillation under reduced pressure, then added ethanol (its amount was 6 times the weight of raw material 1), stirred for 1 hour, and evaporated the solvent; Toluene (the amount is 7 times the weight of the raw material 1), stirred at 60°C for 2 hours, evaporated to remove the solvent to concentrate the material to 2 / 3 volume, cooled to 2-8°C, filtered, and the filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com