Pharmaceutical compositions comprising poh derivatives and methods of use
A composition and drug technology, applied in the field of perillyl alcohol derivatives and isoperillyl alcohol derivatives, can solve the problems of tumor acquired drug resistance and undesired side effects hindering the long-term treatment of chemotherapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
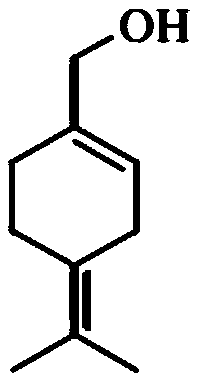
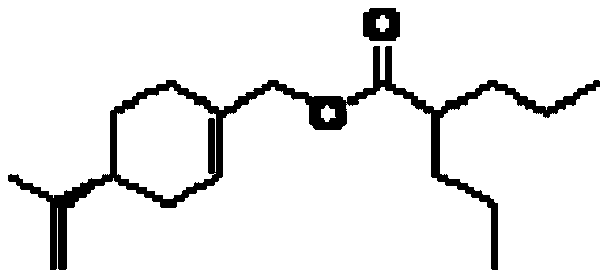
[0124] Example 1: Synthesis of 4-isopropenylcyclohex-1-enylmethyl 2-propylpentanoate (POH-valproate conjugate)
[0125] The reaction scheme is as follows.
[0126]
[0127] Protocol for the synthesis of 4-isopropenylcyclohex-1-enylmethyl 2-propylvalerate (POH-valproate):
[0128] To valproic acid (1, 16.0 g, 110 mmol) was slowly added thionyl chloride (39.6 g, 332 mmol) while maintaining the internal temperature at 10°C. The resulting mixture was allowed to warm to room temperature (RT) and stirred for about 4.0 hours. Excess thionyl chloride was recovered by concentration under vacuum to give valproyl chloride (2) as pale yellow liquid (16.8 g, yield: 94%).
[0129] Valproyl chloride (2, 11.08 g, 68.14 mmol) was slowly added to a mixture of perillyl alcohol (POH 3 , 8.0 g, 52.55 mmol), triethylamine (8.5 g, 84 mmol) and dichloromethane (80 mL), At the same time keep the temperature between 10-15°C. The mixture was stirred at RT for 2.0 h, and then quenched with water (...
Embodiment 2
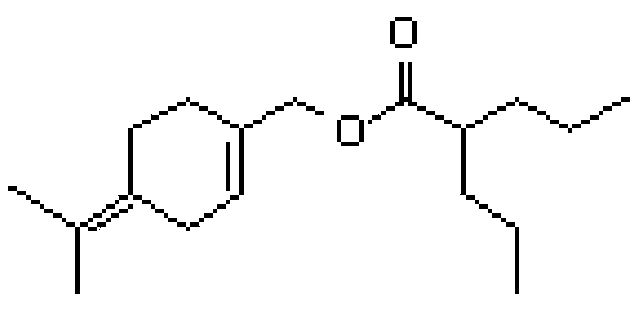
[0130] Example 2: Synthesis of 2-propyl-pentanoic acid 4-isopropylidene-cyclohex-1-enyl methyl ester (isoPOH-valproate conjugate)
[0131] The reaction scheme is as follows.
[0132]
[0133] Protocol for the synthesis of 4-isopropylidene-cyclohex-1-enylmethyl 2-propyl-valerate (isoPOH-valproate):
[0134] To valproic acid (1, 16.0 g, 110 mmol) was slowly added thionyl chloride (39.6 g, 332 mmol) while maintaining the internal temperature at 10°C. The resulting mixture was allowed to warm to room temperature (RT) and stirred for about 4.0 hours. Excess thionyl chloride was removed by concentration under vacuum to give valproyl chloride (2) as pale yellow liquid (16.8 g, yield: 94%).
[0135] Valproyl chloride (2, 11.08 g, 68.14 mmol) was slowly added to a mixture of isoperillyl alcohol (isoPOH 3 , 8.0 g, 52.55 mmol), triethylamine (8.5 g, 84 mmol) and dichloromethane (80 mL) , while maintaining the temperature between 10-15°C. The mixture was stirred at RT for 2.0 h, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com