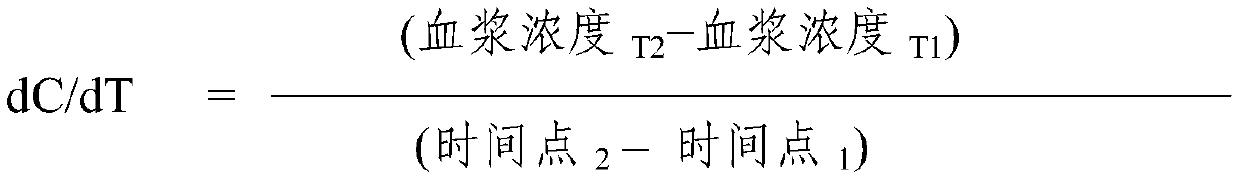
Nasal dosage forms of dihydroergotamine
A dihydroergotamine and ergotamine nasal technology, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, aerosol delivery, etc., can solve the problem that there is no dihydroergotamine nasal dosage form available And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
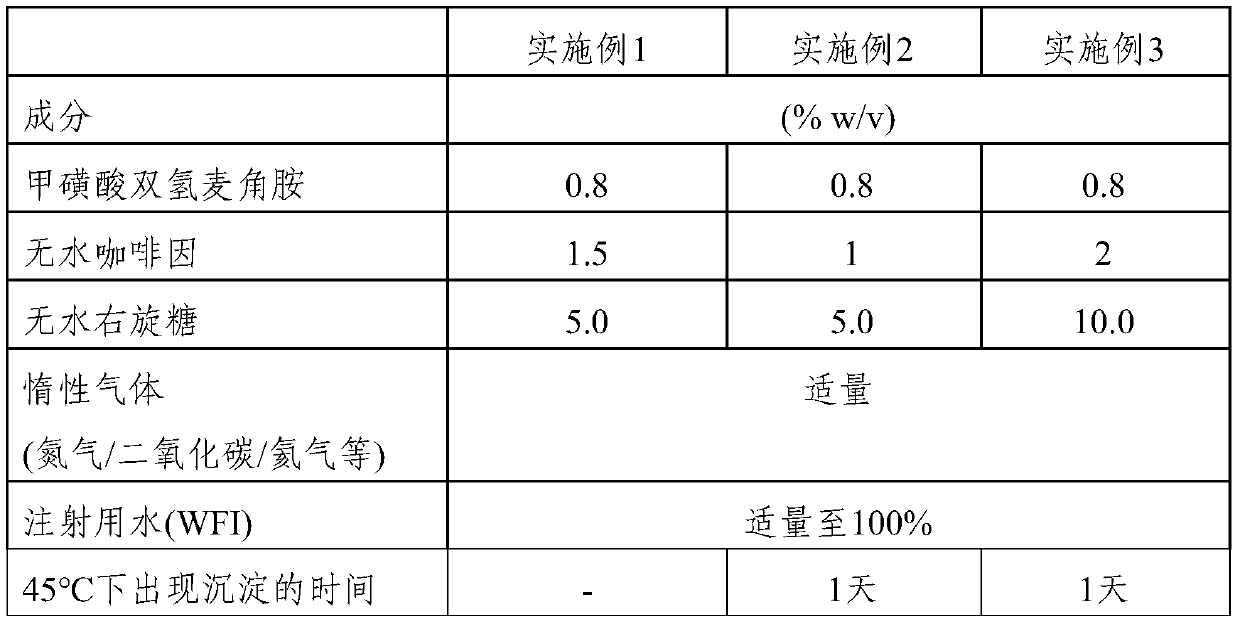
[0404] Compositions of dihydroergotamine mesylate (DHE) were prepared as outlined below.
[0405] The time to precipitation (PPT) of the prepared compositions was evaluated.
[0406] Table 1
[0407]
[0408] Preparation:
[0409] The WFI was bubbled with carbon dioxide and the caffeine, DHE and dextrose were dissolved to obtain a clear solution. The volume of the solution was adjusted to the desired level, then filtered. Store the filtered solution in glass vials and close with rubber stoppers and aluminum seals.
Embodiment 4-5
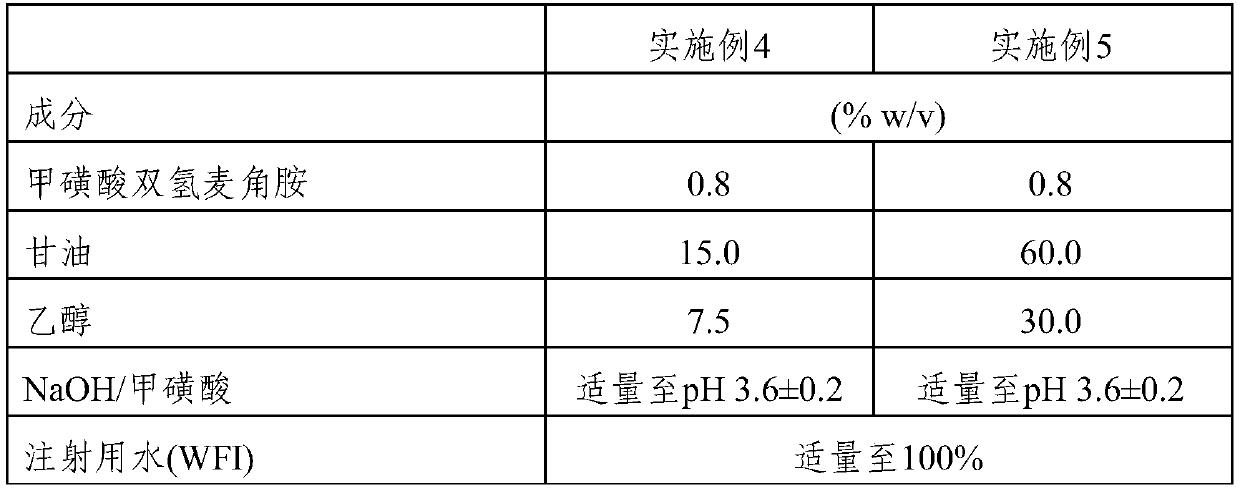
[0411] A composition of dihydroergotamine mesylate was prepared as outlined below. The time to precipitation of the prepared compositions was evaluated.
[0412] Table 2
[0413]
[0414]
[0415] Preparation:
[0416] Add sufficient methanesulfonic acid to the WFI to bring the pH to 3.6 ± 0.2. Dissolve ethanol, glycerol and DHE in WFI to obtain a clear solution. The volume of the solution was adjusted to the desired level, then filtered. Store the filtered solution in glass vials and close with rubber stoppers and aluminum seals.
Embodiment 6-12
[0418] Compositions of dihydroergotamine mesylate containing various stabilizers were prepared as outlined below. The time to precipitation of the prepared compositions was evaluated.
[0419] table 3
[0420]
[0421]
[0422] Preparation:
[0423] Bubble the WFI with nitrogen and dissolve the caffeine, DHE and dextrose to obtain a clear solution. Stabilizers such as methanesulfonic acid, citric acid monohydrate, ammonium acetate, lysine acetate, lysine hydrochloride, ascorbic acid as indicated in the table above are then added. Adjust the volume of the solution to the desired level. The formulation was filtered and the solution was stored in glass vials and closed with rubber stoppers and aluminum seals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com