Fast-release lurasidone hydrochloride tablet and preparation process thereof
A lurasidone hydrochloride tablet and a preparation process technology are applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, drug combinations, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
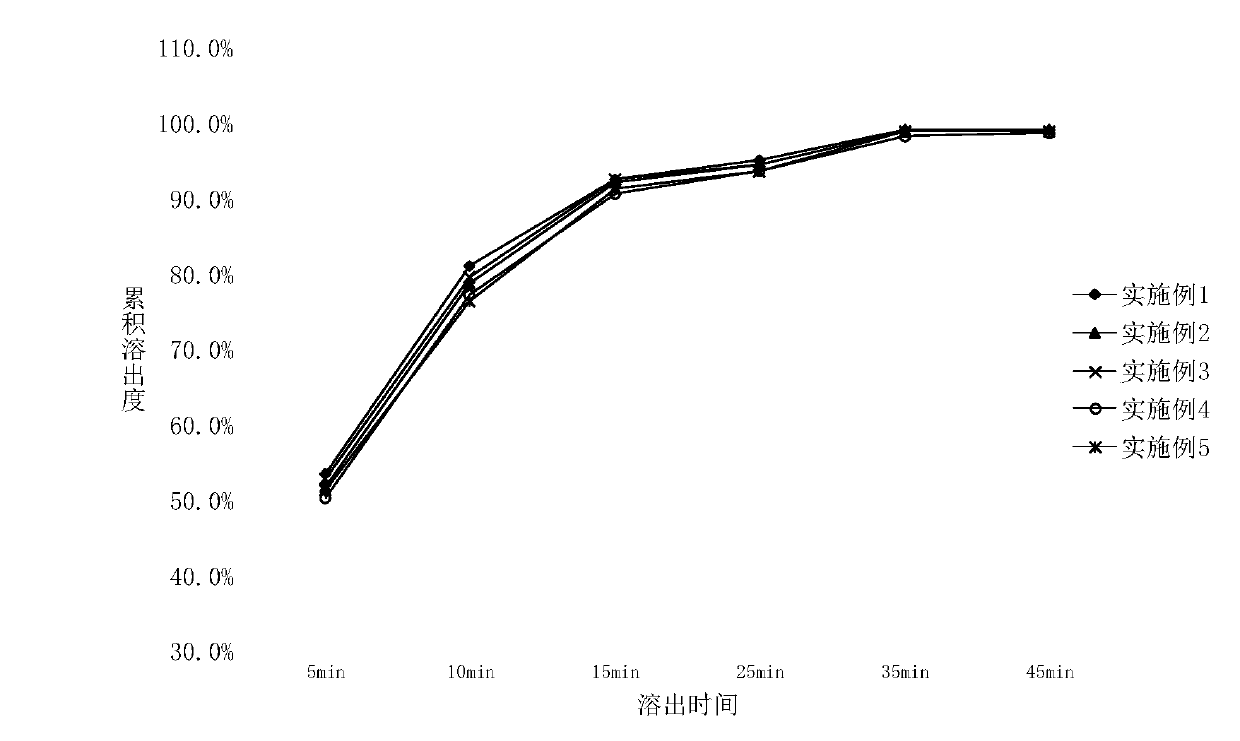
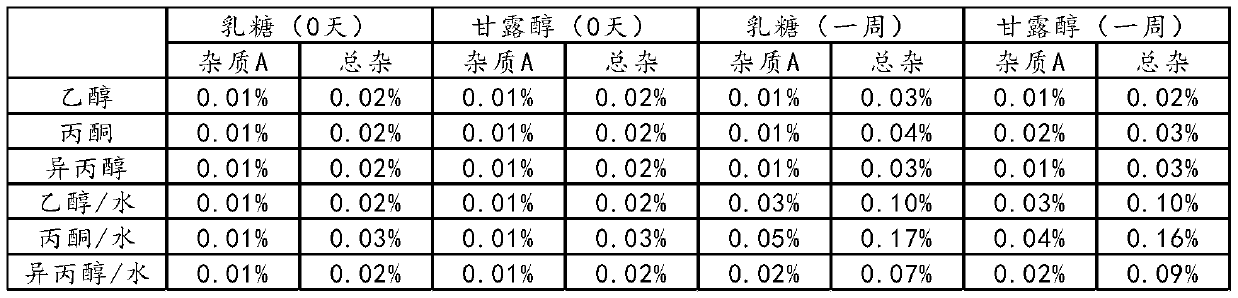
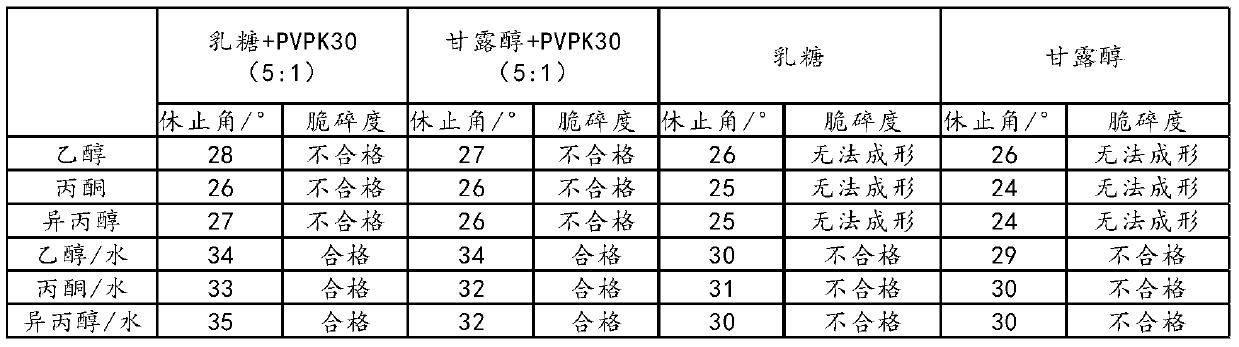
Examples
Embodiment 1
[0039] (1), take lurasidone hydrochloride 20g, mannitol 200g for subsequent use;
[0040] (2) Measure and mix 120mL of isopropanol and 60mL of water, dissolve lurasidone hydrochloride and 40g of mannitol in the above mixture to obtain a uniform and clear single-phase system A;
[0041] (3), 40g PVPK30 and remaining filler are added in the high-speed mixing granulator and mixed uniformly to obtain mixture B;
[0042] (4), adding the single-phase system A in the step (2) to the high-speed mixing granulator in the step (3), and discharging the dry granules after mixing, cutting and drying;
[0043] (5), the obtained tablet of direct compression tablet, totally 1000.
Embodiment 2
[0045] Concrete steps are identical with embodiment 1, and difference is that mannitol total amount is 160g;
Embodiment 3
[0047] Concrete steps are identical with embodiment 1, difference is that the consumption of water is 50mL;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com