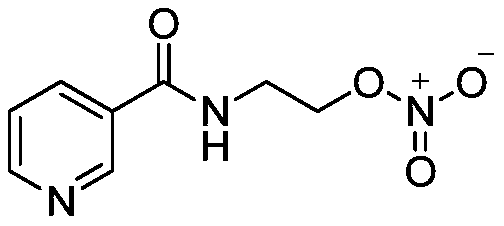
Preparation method of nicorandil
A technology of nicorandil and dilute nitric acid, applied in the direction of organic chemistry, etc., can solve the problems of expensive starting materials, unsatisfactory yields, unstable nitrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
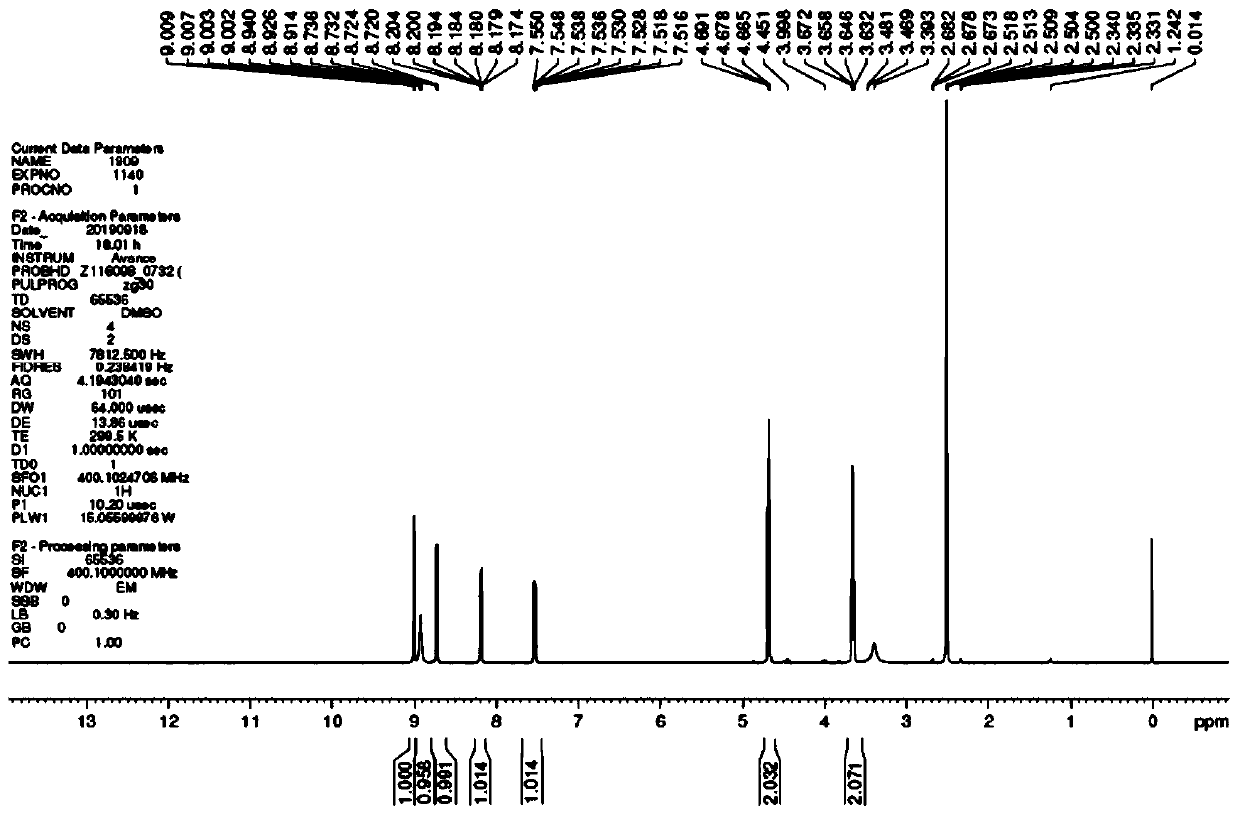
[0056] Embodiment 1: the preparation of nicorandil
[0057] Pour 6.97g (75.22mmol) of 68% dilute nitric acid into a 100ml three-necked reaction flask, cool down in an ice bath to 0-10°C, weigh 17.50g of propionic acid and 12.50g of propionic anhydride into two constant-pressure dropping funnels, Slowly add them dropwise into the reaction system respectively. If the system is exothermic, control the temperature of the reaction system at 0-10°C. The dropwise addition is completed in about 10 minutes, and then keep stirring for 1 hour. Weigh 5.00g (30.088mmol) of N-(2-hydroxyethyl) nicotinic acid amine and add it to the reaction system, remove the ice bath, warm up to 20-25°C, stir and react for 3 hours, and the liquid phase monitors that the remaining raw materials are less than 1.0 %. Stop the reaction, pour the reaction solution into 50ml of 10% dilute ammonia water to disperse and stir, a large amount of white solids precipitated, lower the temperature to 0-10°C, stir and cr...
Embodiment 2
[0058] Embodiment 2. Preparation of Nicorandil
[0059] Take a 100ml three-necked reaction flask and add 8.36g (90.264mmol) of 68% dilute nitric acid, cool down in an ice bath to 0-10°C, weigh 20.00g of propionic acid and 15.00g of propionic anhydride into two constant pressure dropping funnels, respectively Slowly add dropwise to the reaction system, the system will generate heat, control the temperature of the reaction system at 0-5°C, complete the dropwise addition in about 15 minutes, keep stirring for 1.5 hours. Weigh 5.00g (30.088mmol) of N-(2-hydroxyethyl) nicotinic acid amine and add it to the reaction system, remove the ice bath, warm up to 20-30°C, stir and react for 3.5 hours, and the liquid phase monitors that the remaining raw materials are less than 0.5 %. Stop the reaction, pour the reaction solution into 50ml of 12% dilute ammonia water to disperse and stir, a large amount of white solids precipitated, lower the temperature to 0-5°C, stir and crystallize, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com