Pyrrolopyrrole monoketotetrabenzene analogue polymer, preparation method and applications thereof
A technology of pyrrolopyrrole monoketone and pyrrolopyrrole monoketone, which is applied in the field of pyrrolopyrrole monoketopetracene analog polymers and their preparation, can solve the problems of complex reaction steps and low yield, and achieve good dissolution The performance, the synthesis method is simple, and the effect that is conducive to the transmission of electrons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A method for preparing an aza-tetracene analog polymer of pyrrole monoketone, comprising the steps of:
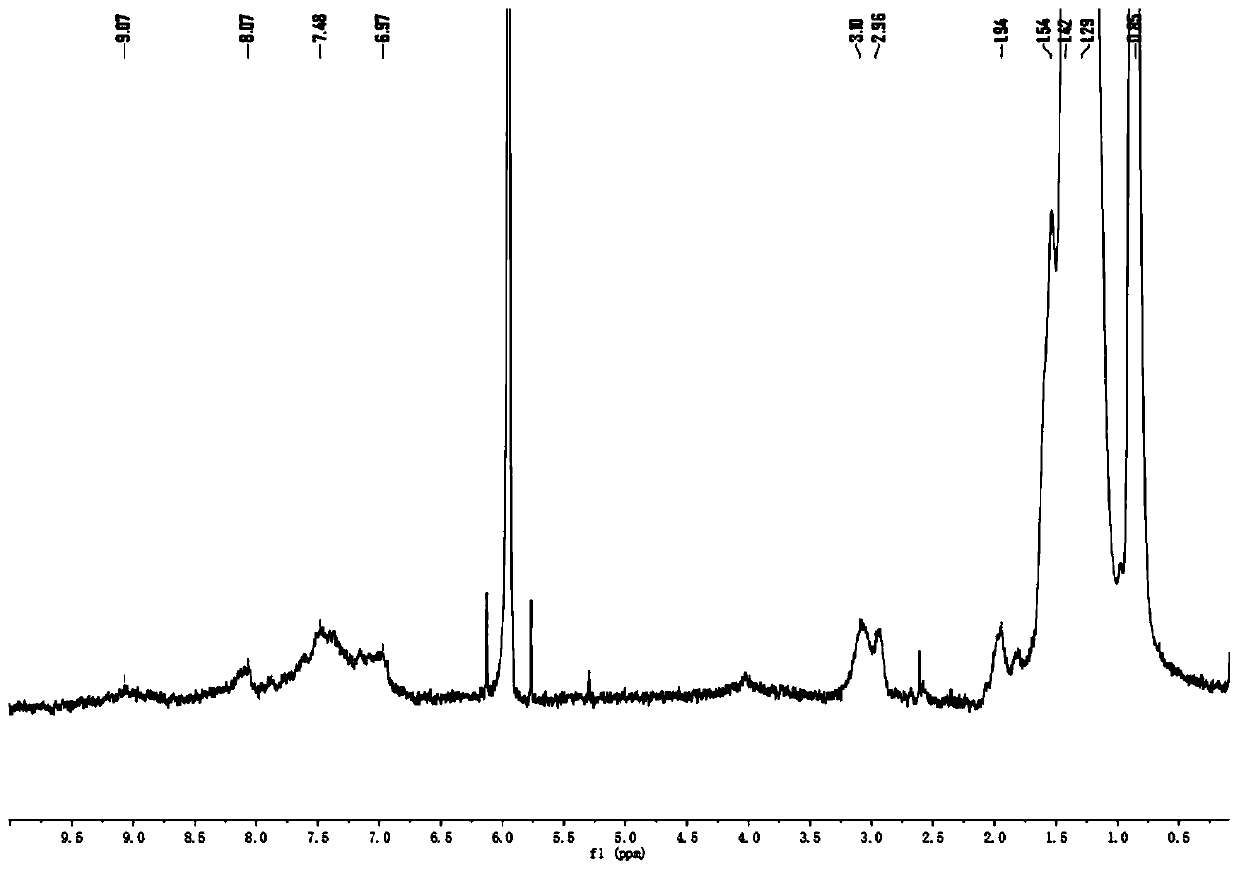
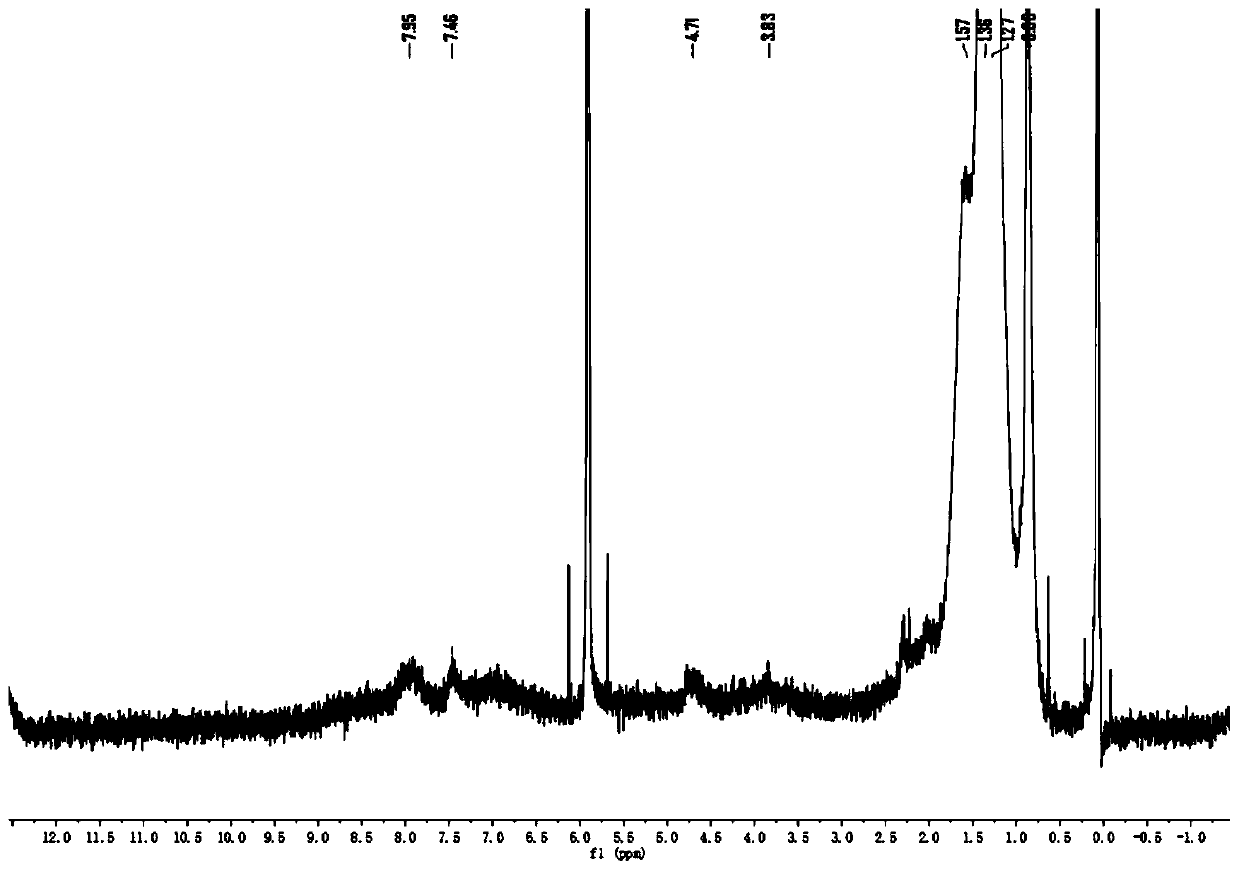
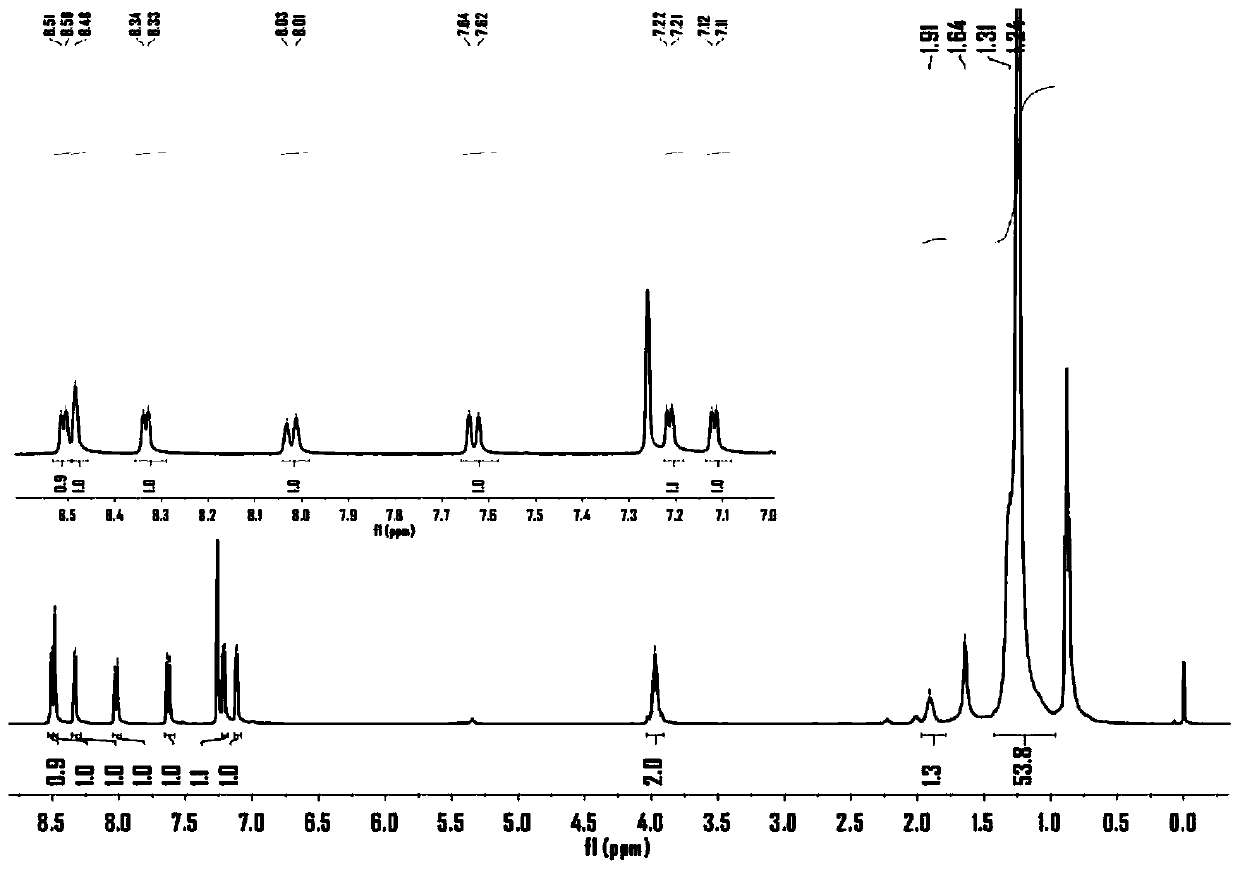
[0060] (1) 3.00g 3,6-di(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (I) and 2.9g tert-butanol Potassium was added to a three-neck round bottom flask, degassed and then filled with nitrogen for three consecutive times, then added 50mL N,N-dimethylformamide (DMF), stirred, and then added 1-bromo-2-decyltetradecane C 24 h 49 Br 4.17g, reacted at room temperature for 4h. The obtained solution was rotary evaporated first, then purified by chromatography column, and compound II could be obtained by distillation under reduced pressure. The quality of the obtained compound was 1.57 g, and the yield was 32%; image 3 for compound II 1 H NMR spectrum, Figure 4 for compound II 13 C NMR, proton nuclear magnetic resonance spectrum can prove that the synthesized compound is structure II.
[0061] (2) Add 1.46g of compound (II), 1.52g of potassium carbonate and 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com