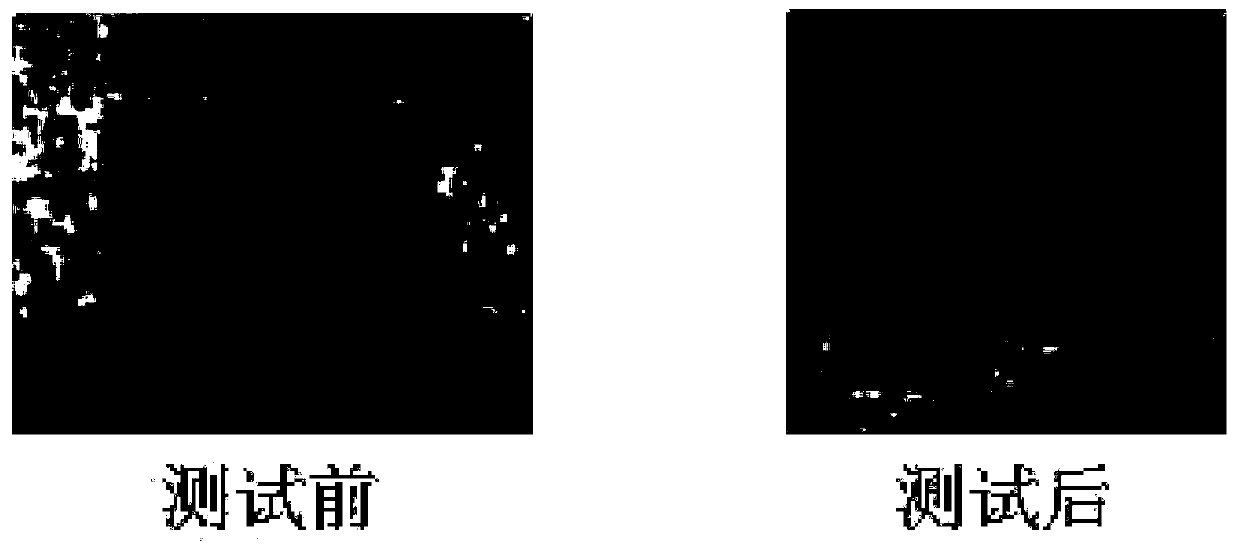
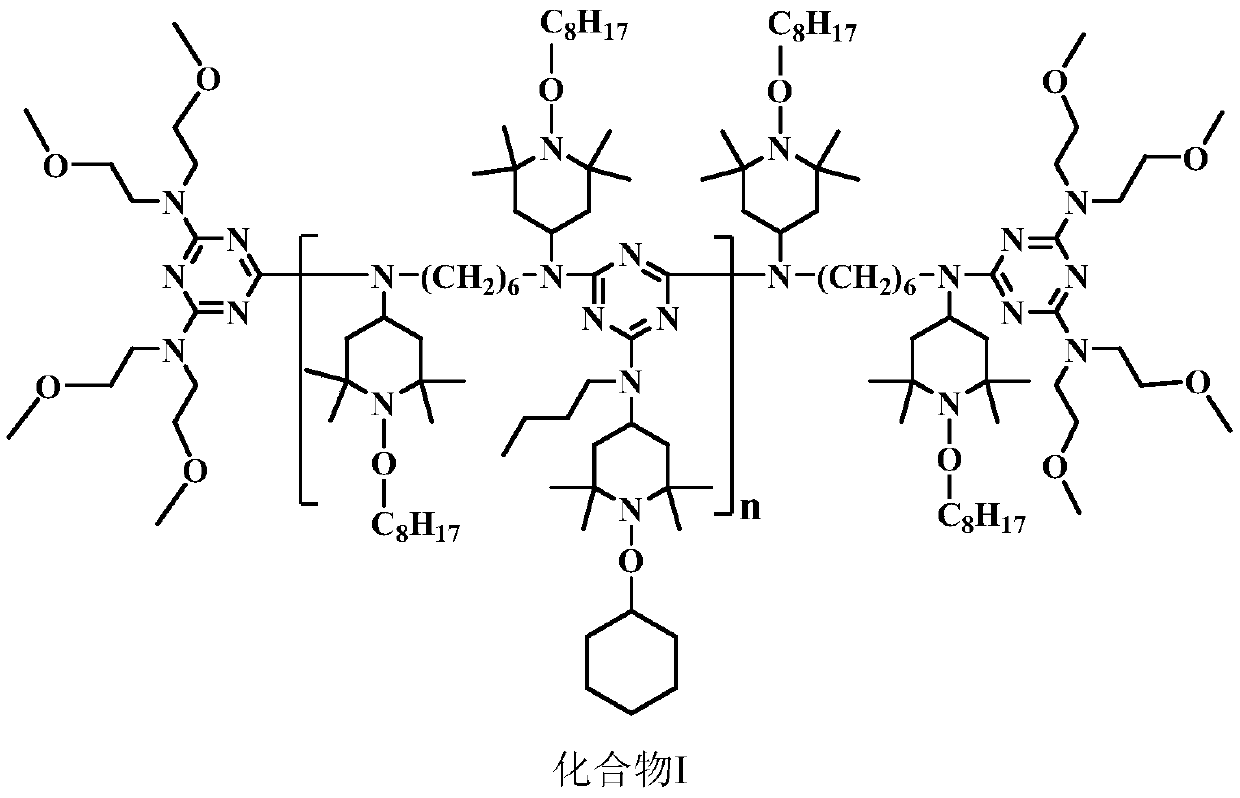
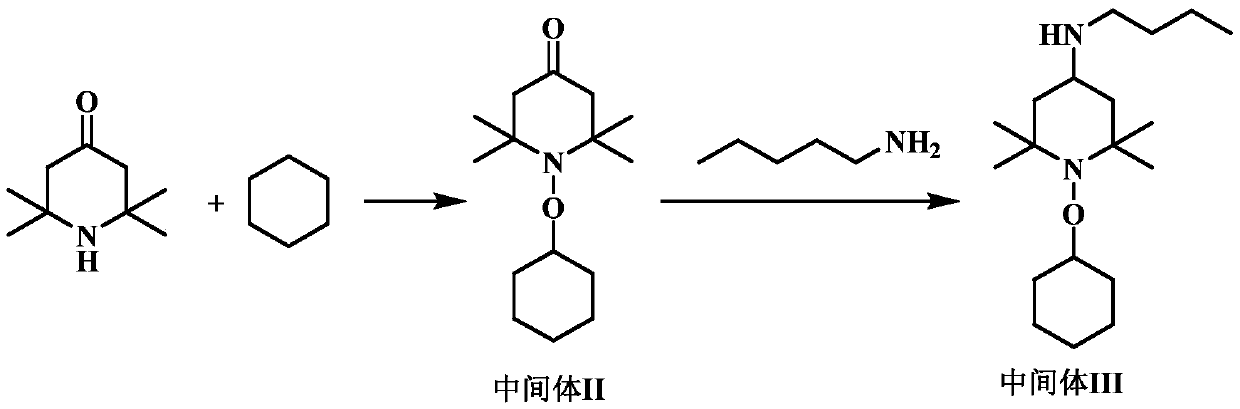
High-molecular-weight low-alkalinity light stabilizer as well as preparation and application thereof
A light stabilizer, high molecular weight technology, applied in high molecular weight low alkali light stabilizer and its preparation and application fields, can solve the problems of low alkalinity and high alkalinity, and achieve mild conditions and excellent light stability performance. , the effect of preventing volatilization and migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Add 155g of 2,2,6,6-tetramethylpiperidone, 0.4g of magnesium hydroxide and 1500mL of water to the four-necked flask in sequence, and heat to 65°C. Nitrogen was used to drive oxygen, and 155mL of 30% hydrogen peroxide was added dropwise under the protection of nitrogen, and the drop was completed within 6 hours, and the reaction was continued for 6 hours after the addition was completed. After the reaction, heat an appropriate amount of chloroform for extraction. The extract is washed several times with dilute hydrochloric acid, water and dilute sodium hydroxide, and finally dried with magnesium sulfate. After filtration, the solvent is evaporated to obtain dark red needle-like crystals. Weigh 17g of needle crystals and dissolve in 50.4g of cyclohexane, dissolve 2.68g of copper bromide and 3.87g of tetrabutylammonium bromide in an appropriate amount of water, mix the two, heat to 80°C, and add dropwise 24.0g of 75% The tert-butyl hydroperoxide is added dropwise within ha...
Embodiment 2
[0068] Add 155g of 2,2,6,6-tetramethylpiperidone, 0.4g of magnesium hydroxide and 1500mL of water to the four-necked flask in sequence, and heat to 65°C. Nitrogen was used to drive oxygen, and 155mL of 30% hydrogen peroxide was added dropwise under the protection of nitrogen, and the drop was completed within 6 hours, and the reaction was continued for 6 hours after the addition was completed. After the reaction, heat an appropriate amount of chloroform for extraction. The extract is washed several times with dilute hydrochloric acid, water and dilute sodium hydroxide, and finally dried with magnesium sulfate. After filtration, the solvent is evaporated to obtain dark red needle-like crystals. Weigh 17g of needle crystals and dissolve in 50.4g of cyclohexane, dissolve 2.68g of copper bromide and 3.87g of tetrabutylammonium bromide in an appropriate amount of water, mix the two, heat to 80°C, and add dropwise 24.0g of 75% The tert-butyl hydroperoxide is added dropwise within ha...
Embodiment 3
[0077] Add 155g of 2,2,6,6-tetramethylpiperidone, 0.4g of magnesium hydroxide and 1500mL of water to the four-necked flask in sequence, and heat to 65°C. Nitrogen was used to drive oxygen, and 155mL of 30% hydrogen peroxide was added dropwise under the protection of nitrogen, and the drop was completed within 6 hours, and the reaction was continued for 6 hours after the addition was completed. After the reaction, heat an appropriate amount of chloroform for extraction. The extract is washed several times with dilute hydrochloric acid, water and dilute sodium hydroxide, and finally dried with magnesium sulfate. After filtration, the solvent is evaporated to obtain dark red needle-like crystals. Weigh 17g of needle-like crystals and dissolve in 50.4g of cyclohexane, 3.12g of ferrous sulfate, 5mL of glacial acetic acid and 3.87g of tetrabutylammonium bromide are dissolved in an appropriate amount of water, mix the two, heat to 80°C, and add dropwise 24.0g of 75% tert-butyl hydrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com