Chimeric antigen receptor T cell expressing IL-6R blocking antibody and targeting CD19, preparation method and application of T cell
A technology of chimeric antigen receptors and expression vectors, which is applied in the fields of genetic engineering and cell biology, can solve the problems of patient death, patient economic burden, and the high price of Tocilizumab, and achieve the goal of reducing the impact of CRS and improving safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of dual expression vectors for CD19 CAR and Tocilizumab
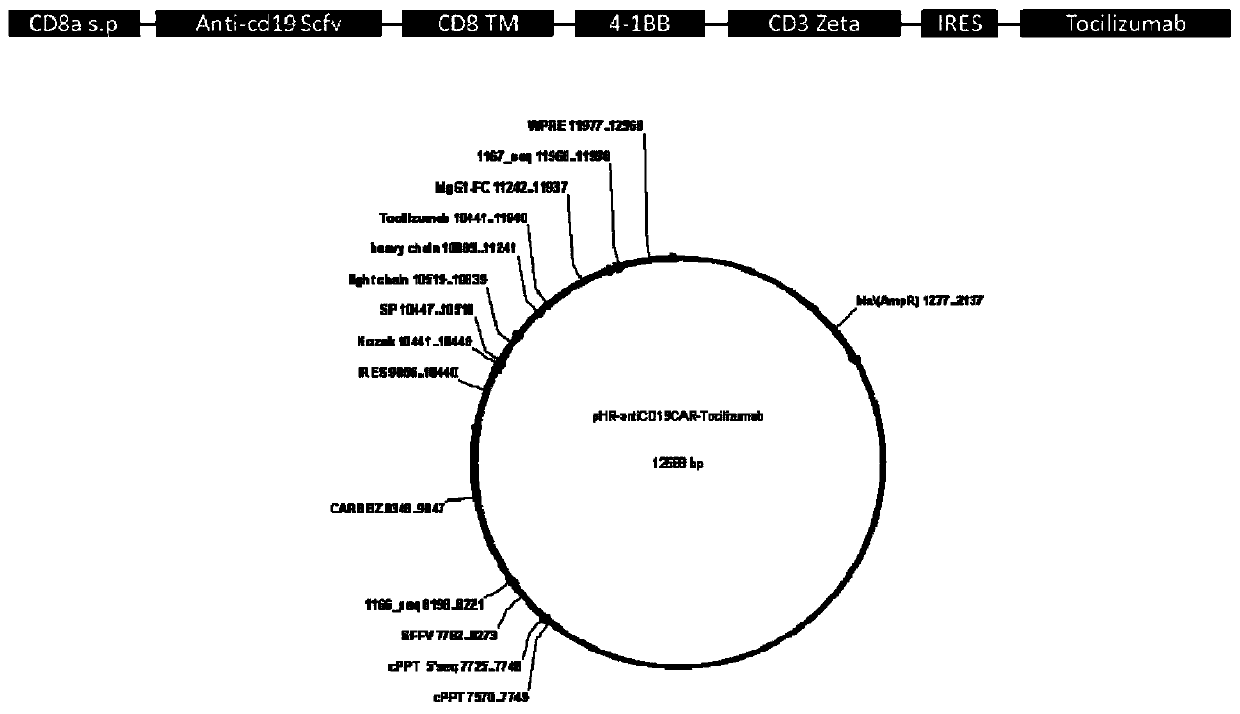
[0052] Such as figure 1 As shown, the CD8 transmembrane signal peptide, anti-CD19 Scfv, CD8 transmembrane region, 4-1BB co-stimulatory signal region, CD3Zeta TCR activation region, IRES, and Tocilizumab were sequentially cloned into the lentiviral backbone plasmid pHR to obtain pHR- antiCD19CAR-Tocilizumab plasmid.
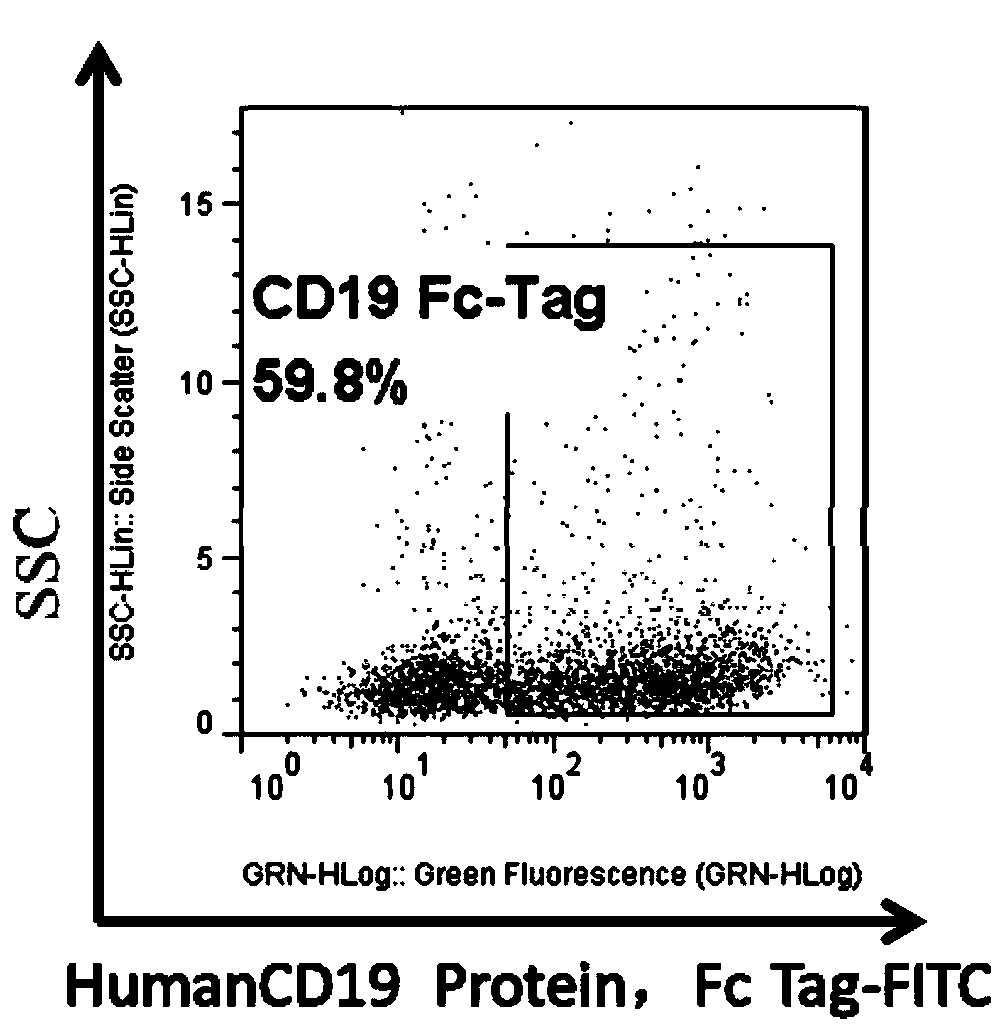
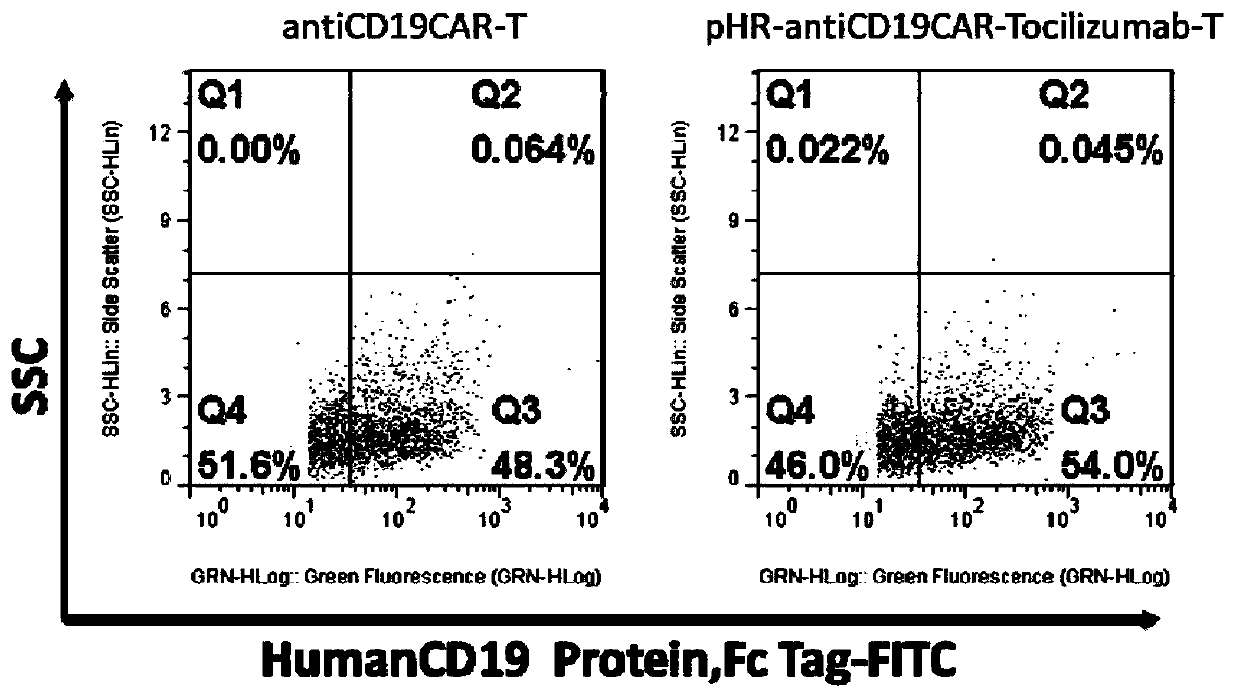
[0053] The nucleotide sequence of antiCD19CAR-Tocilizumab is shown in SEQ ID NO:9, and the amino acid sequence of antiCD19CAR-Tocilizumab is shown in SEQ ID NO:10. Transfect 1ug of the lentiviral expression vector carrying the target gene into 293FT cells, collect 293FT cells 5×105 after 24h, add 10ul of FITC-LabeledHumanCD19 (20-291) Protein to each sample, and incubate for 1h at 4°C in the dark , washed 3 times, centrifuged, and the pellet was resuspended in 200 μL PBS and tested on a machine (Millipore guava easyCyte HT). The transfection efficiency of pHR-antiCD19CAR-To...
Embodiment 2
[0054] Example 2: Packaging and concentration of lentivirus
[0055] The lentiviral expression vector carrying the target gene, pCMV vector and pMD.2G vector were mixed and transfected into 293FT cells. After transfection, 6h-8h was replaced with complete medium for culture. After 48h, the culture medium was collected and centrifuged. Keep the supernatant and filter the supernatant with a 0.45 μm filter, keep the filtrate, and the filtrate is the solution of the recombinant lentivirus.
[0056] Lentivirus concentration was carried out according to the instructions of Lenti-XTM Concentrator (takara, cat: 631231).
Embodiment 3
[0057] Example 3: Preparation of T cells with dual expression vectors of CD19 CAR and Tocilizumab
[0058] Take 50mL of fresh blood and conduct density gradient centrifugation with lymphocyte separation medium (Tianjin Haoyang) to separate mononuclear cells. Resuspend mononuclear cells into CTS TM AIM V TM In SFM medium (GIBCO, product number A3021002), the medium should be supplemented with 5% ICS (GIBCO, A2596101). At the same time, CD3 monoclonal antibody and CD28 monoclonal antibody were added to activate T lymphocytes, and cultured at 37°C with 5% CO2 for 48 hours.
[0059] Take 2×106 cells, add the concentrated lentivirus in Example 2 according to MOI=5, add IL-2 and polybrene at the same time, mix well, incubate at 37°C 5% CO2 for 6-8 hours, centrifuge at 300g for 5 minutes to replace the fresh medium CTS TM AIM V TM SFM medium (containing IL-2).
[0060] Add fresh CTS every 2-3 daysTM AIM V TM SFM medium (containing IL-2), maintain the cell density at abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com