Interferon primed plasmacytoid dendritic cells
A plasma cell and cell technology, applied in the field of interferon-induced plasmacytoid dendritic cells, to achieve the effect of improving pDC survival rate and high cell yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] Generate Pdc
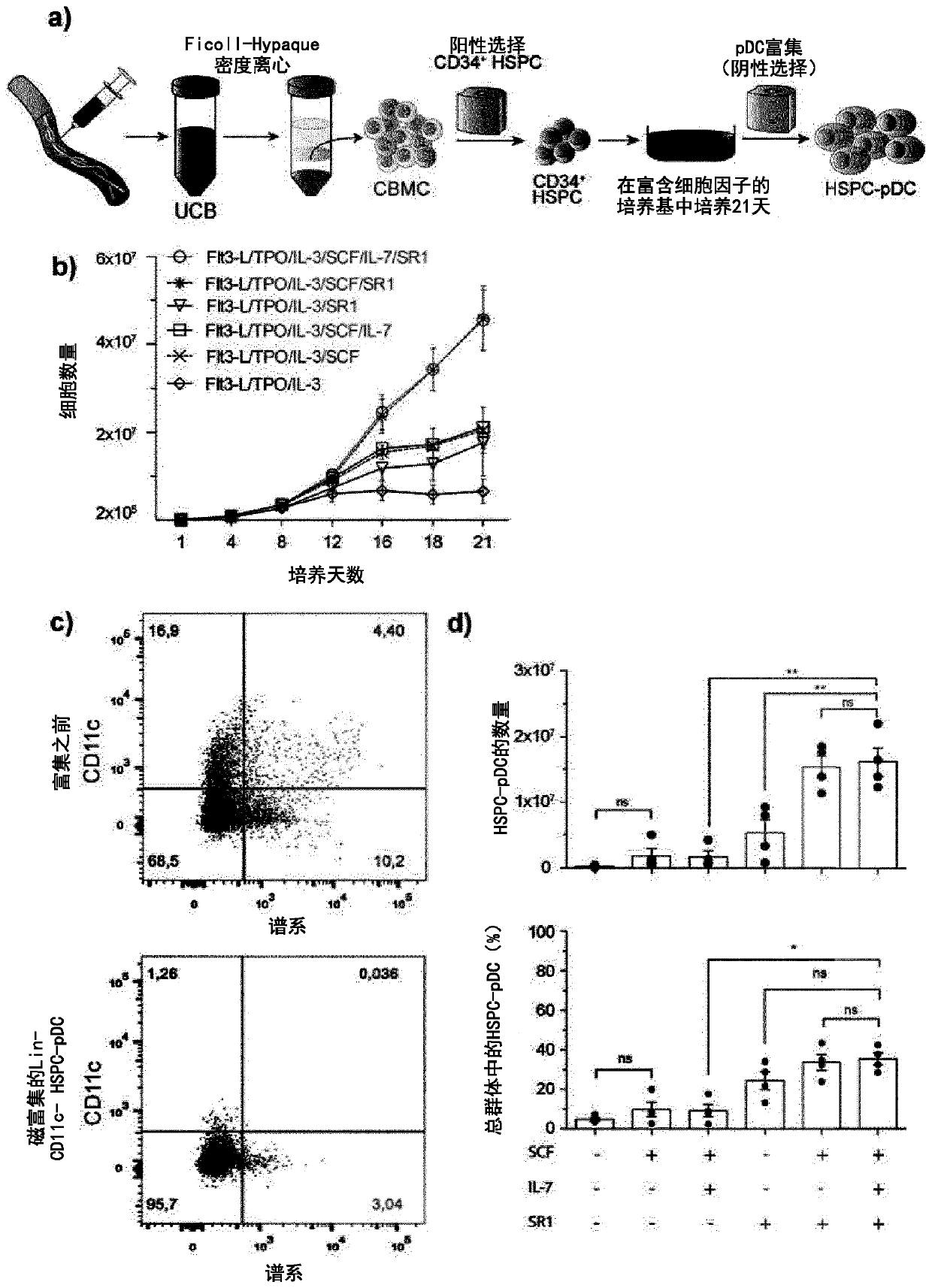
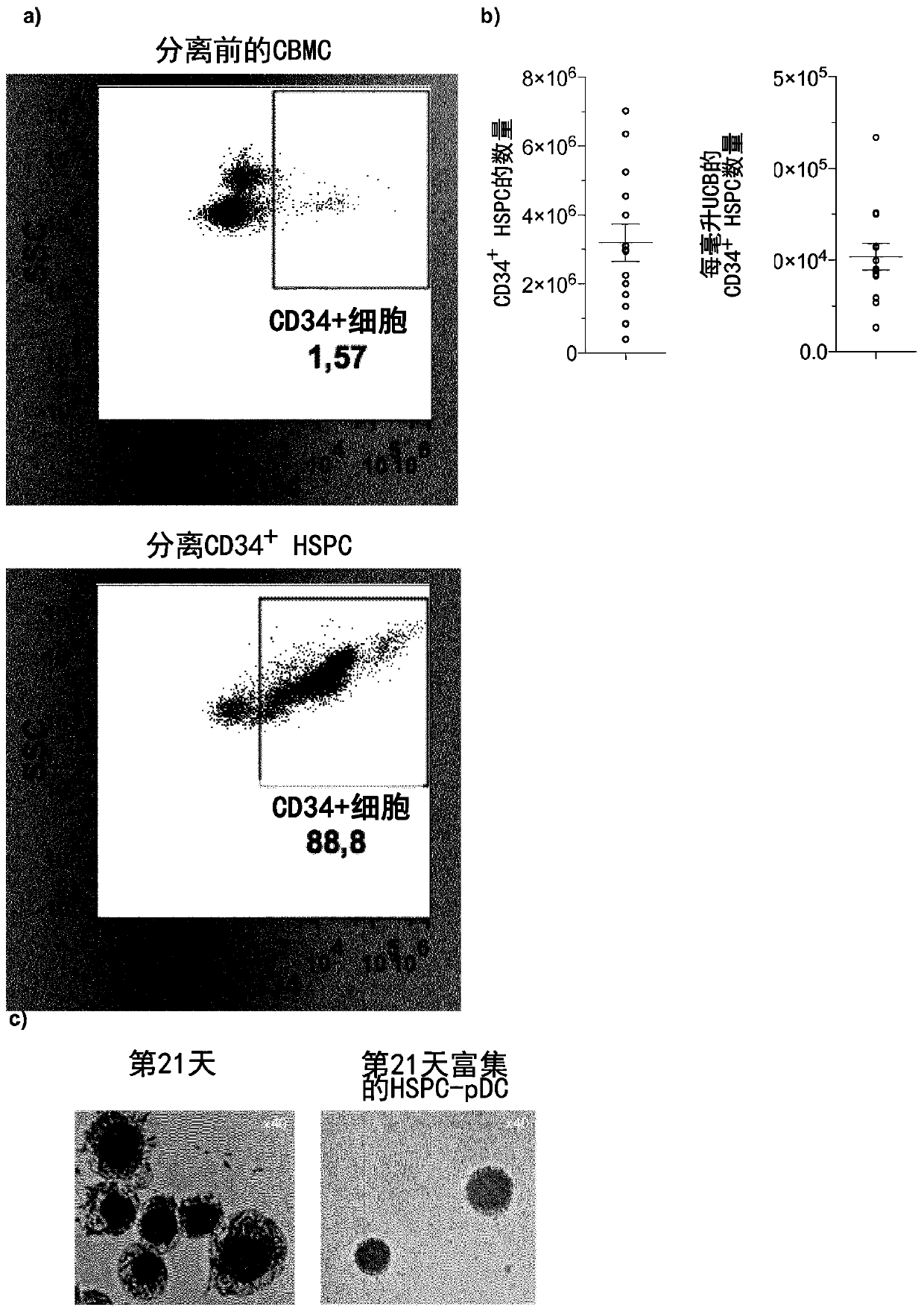
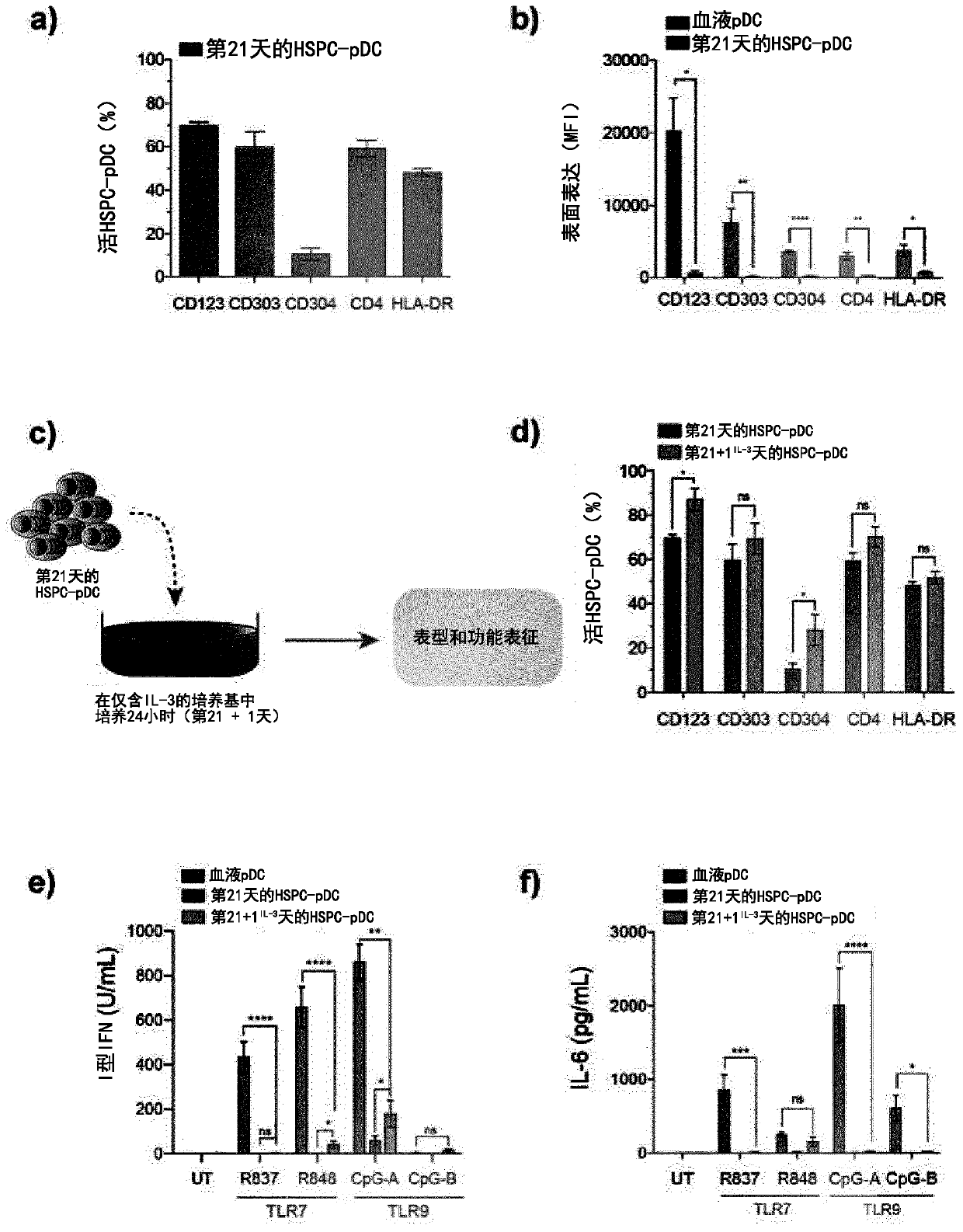
[0268] To validate the experimental approach, results from best practices were reproduced and compared directly [7-10]( figure 1 a). As baseline conditions, a previously reported pDC differentiation protocol requiring 21 h in media containing the cytokines and growth factors Flt3 ligand (Flt3-L), thrombopoietin (TPO), and interleukin 3 was implemented. days [7]. Addition of stem cell factor (SCF) and StemRegenin 1 (SR1) resulted in greater expansion of progenitor cells (240-fold ± 42 compared to 35-fold ± 26 in baseline culture; figure 1 b). Blood-derived pDCs lack expression of lineage-specific surface markers (ie, CD3, CD14, CD16, CD19, CD20, and CD56) and the conventional DC marker CD11c [1]. Therefore, to evaluate the 21-day in vitro differentiation protocol defined as lin neg CD11c neg The number of putative pDCs of the cells was enriched for differentiated pDCs by immunomagnetic negative selection, hereinafter referred to as HSPC-pDCs ( figur...
Embodiment 2
[0277] Production of APCs for therapeutic use
[0278] In addition to producing type I IFN, pDCs can also act as antigen-presenting cells (APCs). One way to use our technology is in anti-tumor immunotherapy. Hematopoietic stem and progenitor cells (HSPCs) are first extracted from a patient's blood sample. HSPCs are then cultured under specific conditions to become pDCs (HSPC-pDCs). HSPC-pDC were then primed for maturation. The primed HSPC-pDCs were then activated with the inactive virus vaccine strain FSME while loading tumor antigens on them. This will induce an antigen-presenting phenotype in HSPC-pDC. Tumor antigens can be obtained directly or indirectly from cancer cells, depending on what is known about the particular cancer type. Direct access requires the loading of specific known tumor antigens onto pDCs by using liposomal nanocarriers. Indirect loading involves cancer cell isolation and co-culture of irradiated cancer cells with FSME-activated pDCs, resulting in...
Embodiment 3
[0285] Generation of genetically modified HSPC-pDC
[0286] Although it is currently not possible to genetically manipulate pDC, advances in CRISPR / Cas9 technology have led to successful and efficient targeting of CD34 + HSPCs for gene editing. Therefore, we speculate that CD34 can be initially modified by + HSPCs were then differentiated into pDCs to successfully generate gene-edited pDCs. We employed a gene editing strategy in which chemically modified synthetic sgRNA and recombinant Cas9 protein (forming a ribonucleoprotein (RNP) complex) were delivered to CD34 by electroporation + HSPC ( Figure 18 a). The sgRNA was designed to target the open reading frame and MyD88 within the first exon of IFNAR1 (subunit of the type I IFN receptor). IFNAR1 was chosen because of its role in TLR-mediated responses, while MyD88 was included because of its established role in TLR-mediated responses. A previously published sgRNA targeting the safe harbor gene CCR5 was used as a nega...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap