Application of mulberroside A and derivatives thereof in preparation of drug for protecting intestinal barrier
A technology of intestinal barrier and derivatives, applied in the field of medicine, can solve the problems of mulperiside A regulating intestinal flora disorder, neuroinflammation, etc., and achieve the effect of improving intestinal barrier damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Effect of Morbariside A on Neuroinflammation in the Hippocampus of High-Fructose Diet Mice
[0027] Experimental method: C57BL / 6N mice were randomly divided into normal group (n=15) and model group (n=60). The normal group was given normal diet, and the model group was given fructose-containing diet. Other feeding conditions were the same for both groups. After 4 weeks, the mice in the model group were randomly divided into 4 groups, namely the fructose model group (n=15), the 20mg / kg mulberryside A administration group (n=15), the 40mg / kg mulberryside A administration group (n=15) and 20 mg / kg resveratrol administration group (n=15). The model group continued to be fed with fructose feed, while the mulberryside A administration group and the resveratrol administration group were fed with fructose feed at the same time, the suspension of mulberryside A or resveratrol was made into normal saline, and then perfused. Stomach administration, gavage once a day. A...
Embodiment 2
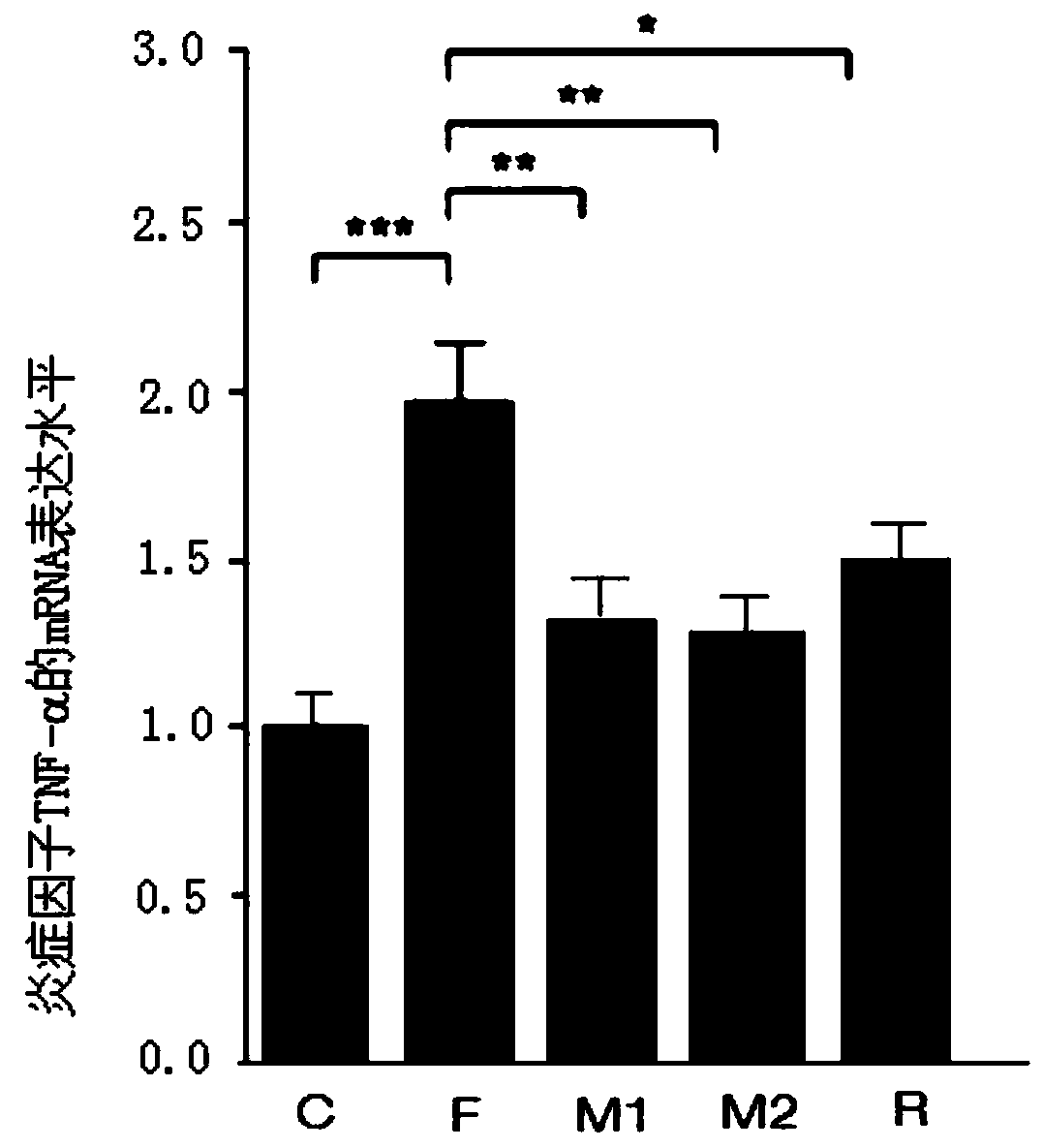
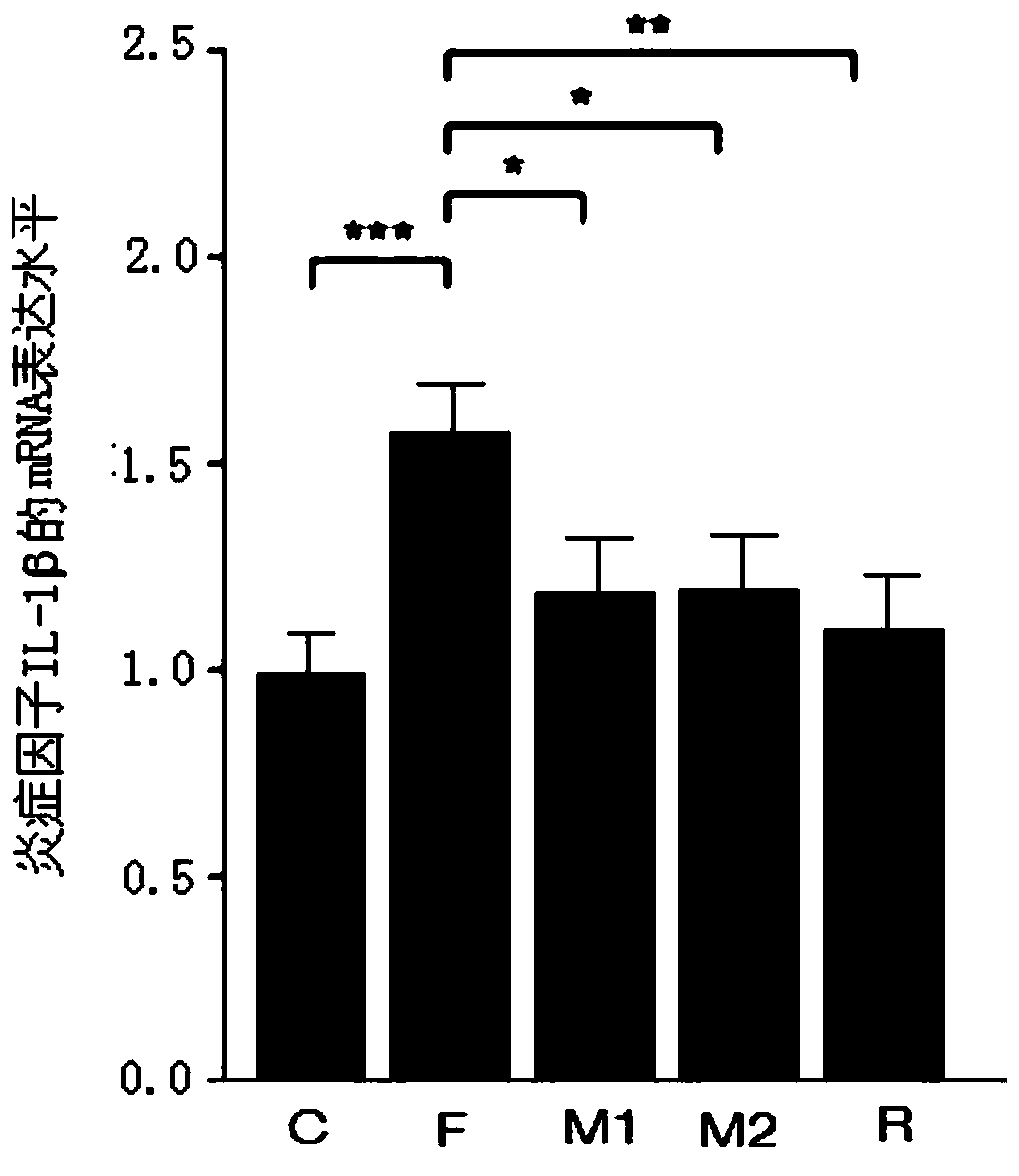
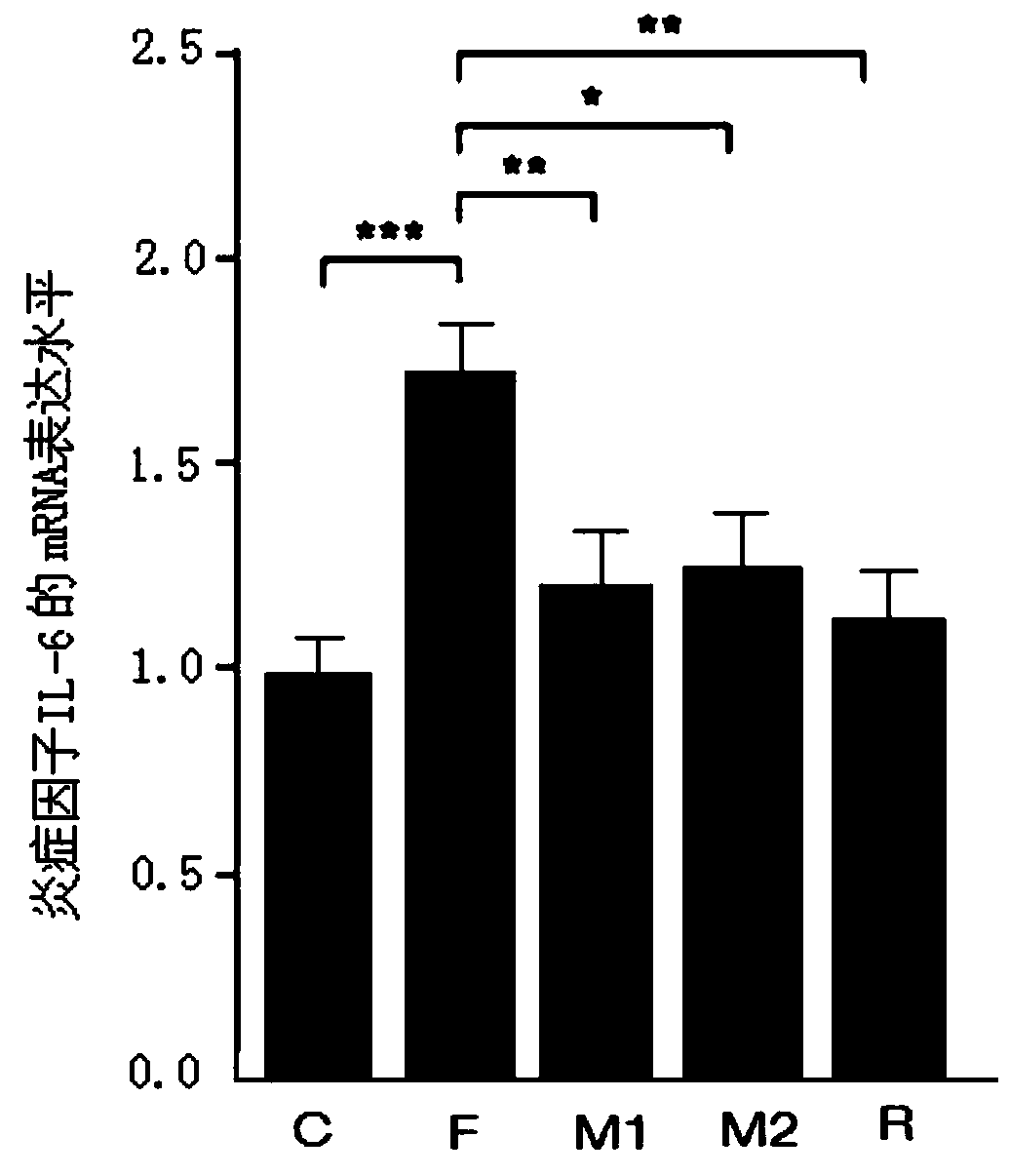
[0030] Example 2 Effect of Morbariside A on Intestinal Barrier Damage in High-Fructose Diet Mice
[0031] See the experimental method above: C57BL / 6N mice were randomly divided into normal group (n=15) and model group (n=60). The normal group was given normal feed, and the model group was given fructose-containing feed. Other feeding conditions were the same for both groups. After 4 weeks, the mice in the model group were randomly divided into 4 groups, namely the fructose model group (n=15), the 20mg / kg mulberryside A administration group (n=15), the 40mg / kg mulberryside A administration group (n=15) and 20 mg / kg resveratrol administration group (n=15). The model group continued to be fed with fructose feed, while the mulberryside A administration group and the resveratrol administration group were fed with fructose feed at the same time, the suspensions made of mulberryside A or resveratrol with normal saline were fed. Stomach administration, gavage once a day. After 8 wee...
Embodiment 3
[0035] Example 3 Effects of Morin A on intestinal flora disorder of high fructose diet mice
[0036]See the experimental method above: C57BL / 6N mice were randomly divided into normal group (n=15) and model group (n=60). The normal group was given normal feed, and the model group was given fructose-containing feed. Other feeding conditions were the same for both groups. After 4 weeks, the mice in the model group were randomly divided into 4 groups, namely the fructose model group (n=15), the 20mg / kg mulberryside A administration group (n=15), the 40mg / kg mulberryside A administration group (n=15) and 20 mg / kg resveratrol administration group (n=15). The model group continued to be fed with fructose feed, while the mulberryside A administration group and the resveratrol administration group were fed with fructose feed at the same time, the suspension of mulberryside A or resveratrol was made into normal saline, and then perfused. Stomach administration, gavage once a day. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com