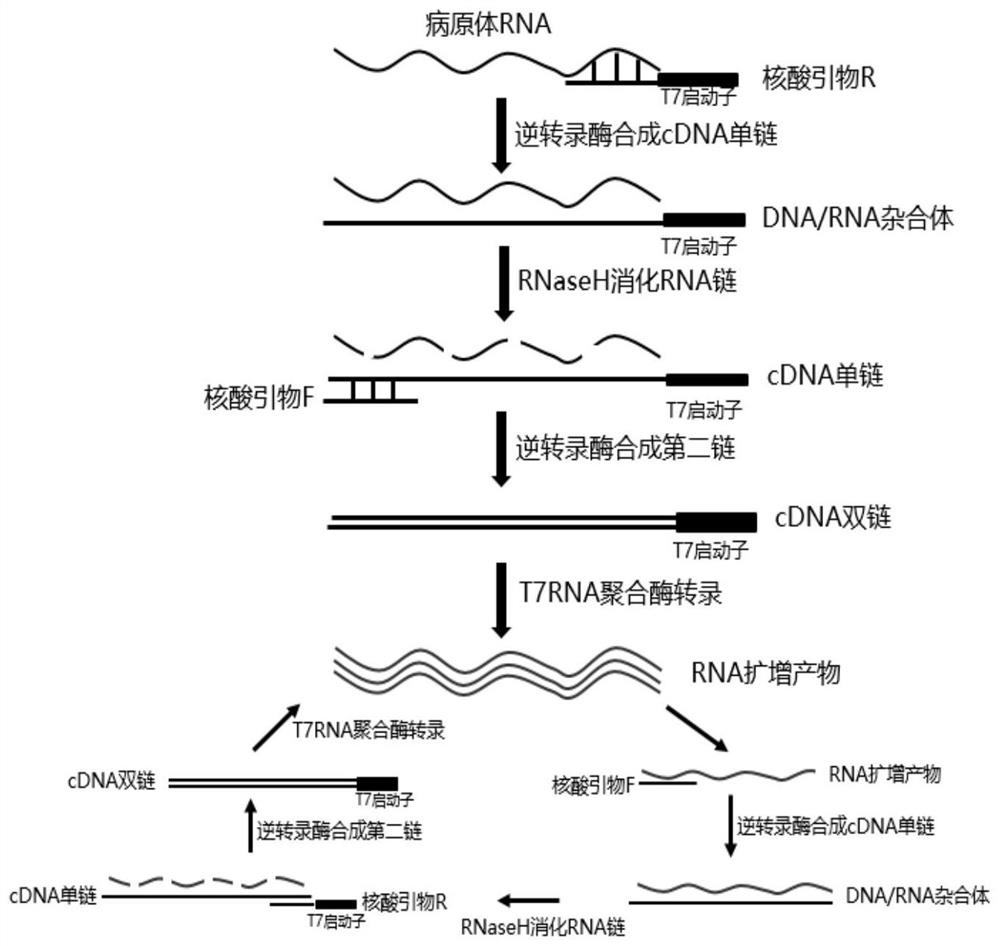
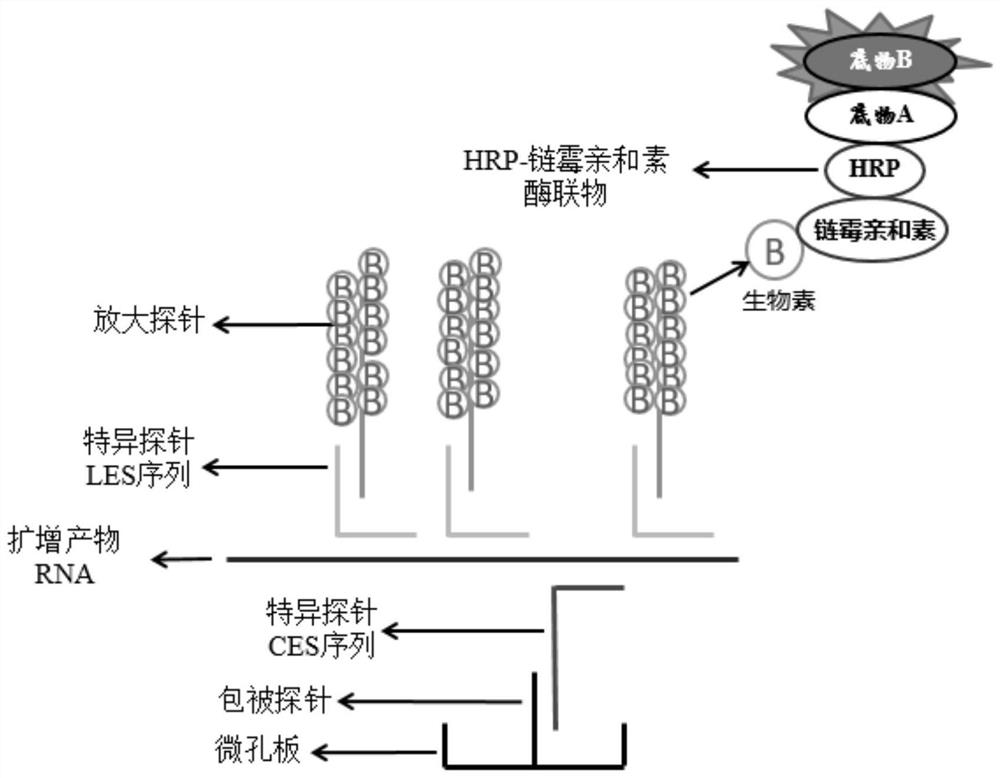
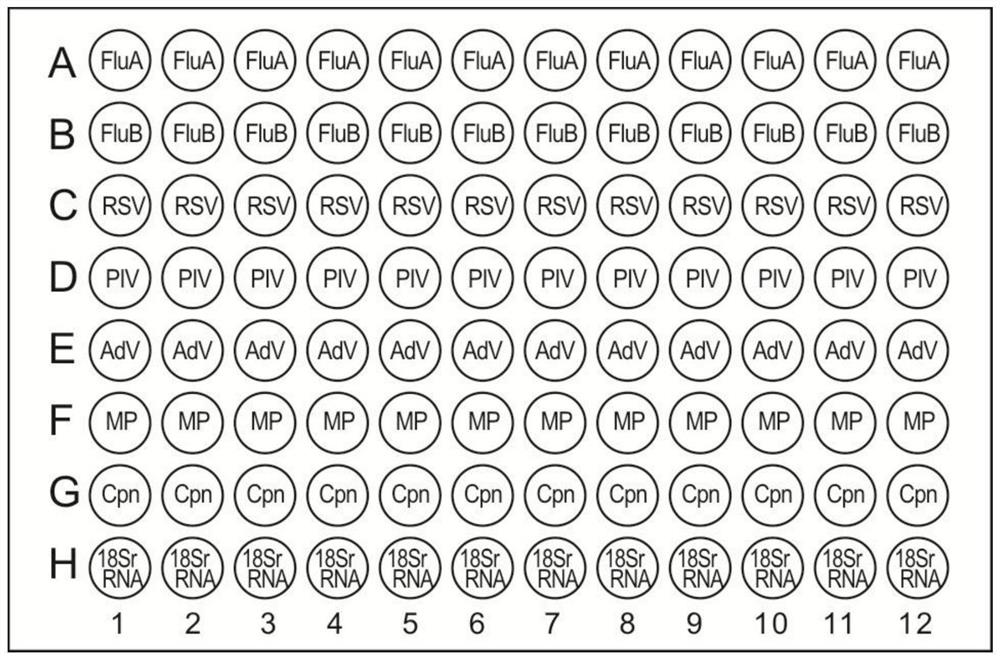
Kit for simultaneous detection of nucleic acids of seven respiratory pathogens and application thereof
A technology for respiratory tract and pathogens, applied in the field of kits for joint detection of seven respiratory pathogenic nucleic acids based on double amplification technology, which can solve the problems of easy pollution and achieve the effects of preventing pollution, reducing requirements, and high amplification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] [Example 1] Sensitivity Test
[0153] The minimum detection limit is determined by gradient dilution of the virus stock solution derived from ATCC. Each gradient virus dilution is repeated 3 to 5 times, and each copy is repeated 20 times. The positive detection rate of 90% to 95% will be determined. virus level as the minimum detection limit,
[0154] The pathogen information is as follows:
[0155] Table 1 Pathogen information
[0156] Subtype ATCC number Corresponding unit H1N1 VR-1469 8.89×10 7 TCID 50 / mL
H3N2 VR-1680D 3.1x 10 6 TCID 50 / mL
FluB VR-1735 2.81×10 7 TCID 50 / mL
RSVA VR-26 1.58×10 7 TCID 50 / mL
RSVB VR-1580 8.89×10 5 TCID 50 / mL
PIV1 VR-94 1.58×10 4 TCID 50 / mL
PIV2 VR-92 2.8×10 6 TCID 50 / mL
PIV3 VR93 1.58×10 7 TCID 50 / mL
AdVB VR-3 1.58×10 8 TCID 50 / mL
AdVE VR-1572 2.8×10 6 TCID 50 / mL
...
Embodiment 2
[0231] [Example 2] Specificity Verification
[0232] 1. Test Strains
[0233] After extracting nucleic acids from different microorganisms, they are tested to verify the specificity of the design of the primers and probes of the kit of the present invention. Relevant pathogens and titers are as follows:
[0234] Table 27 Specificity verification test strain information
[0235]
[0236] 2. Test results
[0237] The test results are as follows:
[0238] Table 28 Specificity Verification Test Results
[0239]
[0240]
[0241] 3. Conclusion
[0242] As can be seen from the above data, the detection results of the kit of the present invention for these microorganisms are all negative, which proves that there is no cross-reaction between the kit of the present invention and other microorganisms, reflecting the strong specificity of the kit for detecting pathogens.
Embodiment 3
[0243] [Example 3] Validation of pathogen detection capability
[0244] Use the kit of the present invention to detect 29 strains of pathogens, and verify the ability of the kit to detect different strains of pathogens. The test results are as follows:
[0245] Table 29 Detection results of different pathogen strains
[0246]
[0247]
[0248] It can be seen from the above results that the kit has good detection ability for different pathogen strains.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com