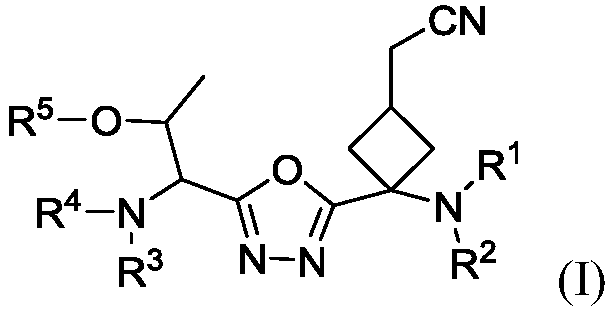
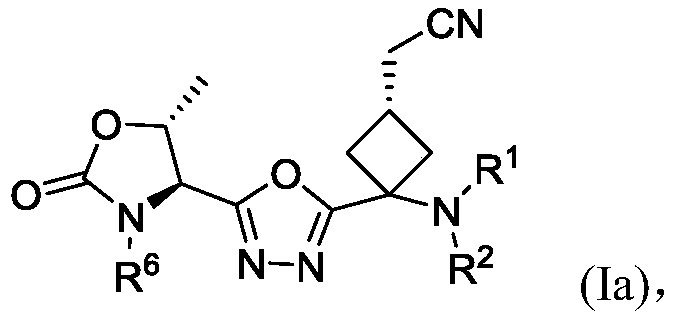
1, 3, 4-oxadiazole-2-cyclobutyl compound and preparation method thereof
A compound and heterocyclic group technology, applied in the field of medicinal chemistry, can solve the problems of immunogenic drug administration route restrictions and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
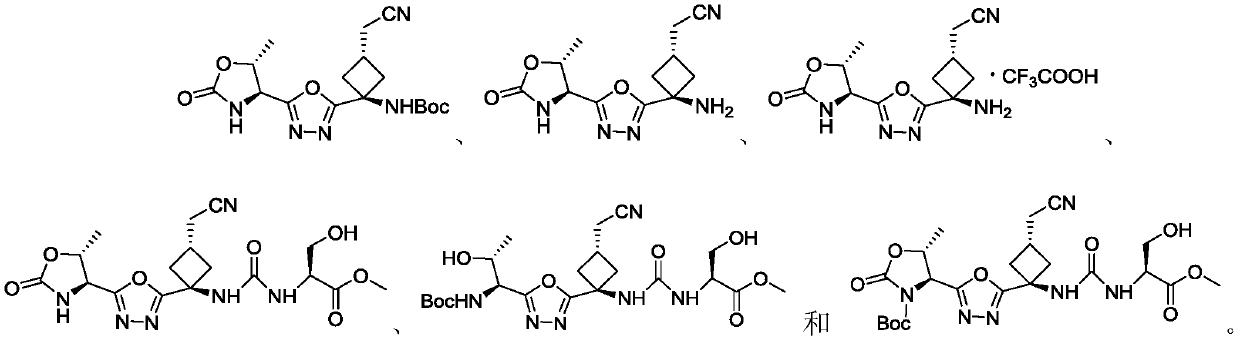
[0084] Example 1: ((1r,3S)-3-(cyanomethyl)-1-(5-((4S,5R)-5-methyl-2-oxooxazolidinone-4-yl)- 1,3,4-oxadiazol-2-yl)cyclobutyl) tert-butyl cyanoformate
[0085]
[0086] Step 1: Preparation of (4S,5R)-5-methyl-2-oxooxazolidinone-4-carboxylic acid
[0087]
[0088] To the sodium hydroxide (1mol / L, 450mL) solution of L-threonine (15.0g, 125.92mmol), slowly drop three (trichloromethyl) carbonate (37.4g, 125.92mmol) of 1,4- Dioxane (300 mL) solution was stirred at room temperature for 6 hours after dropping, concentrated to dryness under reduced pressure, added hot acetonitrile (450 mL) and stirred, filtered, and the filtrate was concentrated to dryness to obtain the title compound (18.1 g, yield 99.0%). m / z calcd for C 5 h 7 NO 4 [M+H] + 146.0, found 146.1.
[0089] Step 2: Preparation of benzyl 2-((4S,5R)-5-methyl-2-oxooxazolidinone-4-carbonyl)hydrazine-1-carboxylate
[0090]
[0091] Under nitrogen protection, at -10°C, drop successively into a solution of (4S,5R)-...
Embodiment 2
[0116] Example 2: 2-((1S,3r)-3-amino-3-(5-((4S,5R)-5-methyl-2-oxooxazolidinone-4-yl)-1, 3,4-Oxadiazol-2-yl)cyclobutyl)acetonitrile trifluoroacetate
[0117]
[0118] Under nitrogen protection, to ((1r,3S)-3-(cyanomethyl)-1-(5-((4S,5R)-5-methyl-2-oxooxazolidinone-4-yl) -1,3,4-oxadiazol-2-yl)cyclobutyl) tert-butyl cyanoformate (1.5g, 3.97mmol) in dichloromethane (15mL) solution was added dropwise trifluoroacetic acid (4.5g, 39.70 mmol), stirred at room temperature for 2 h after dropping, and monitored the end point of the reaction by TLC. Concentrate to dryness under reduced pressure, and the resulting residue is purified by column chromatography to obtain the title compound (1.2 g, 77.3%). 1 HNMR (D 2 O,400MH Z )δ5.02~5.07(m,2H), 3.03~3.13(m,1H), 2.76~2.77(d,J=4Hz, 2H), 2.59~2.67(m,4H), 1.59~1.61(d,J =8Hz,3H); m / z calcd for C 12 h 15 N 5 o 3 [M+H] + 278.12, found 278.2.
Embodiment 3
[0119] Example 3: 2-((1S,3r)-3-amino-3-(5-((4S,5R)-5-methyl-2-oxooxazolidinone-4-yl)-1, 3,4-oxadiazol-2-yl)cyclobutyl)acetonitrile
[0120]
[0121] The preparation method was similar to that of Example 2, except that the amount of trifluoroacetic acid was reduced to obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com