Immunoglobulin binding protein, and affinity support using same
An immunoglobulin and protein technology, applied in the field of immunoglobulin-binding proteins, can solve the problems of difficult antibody purification and low antibody purification efficiency, and achieve high binding activity and high antibody dissociation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
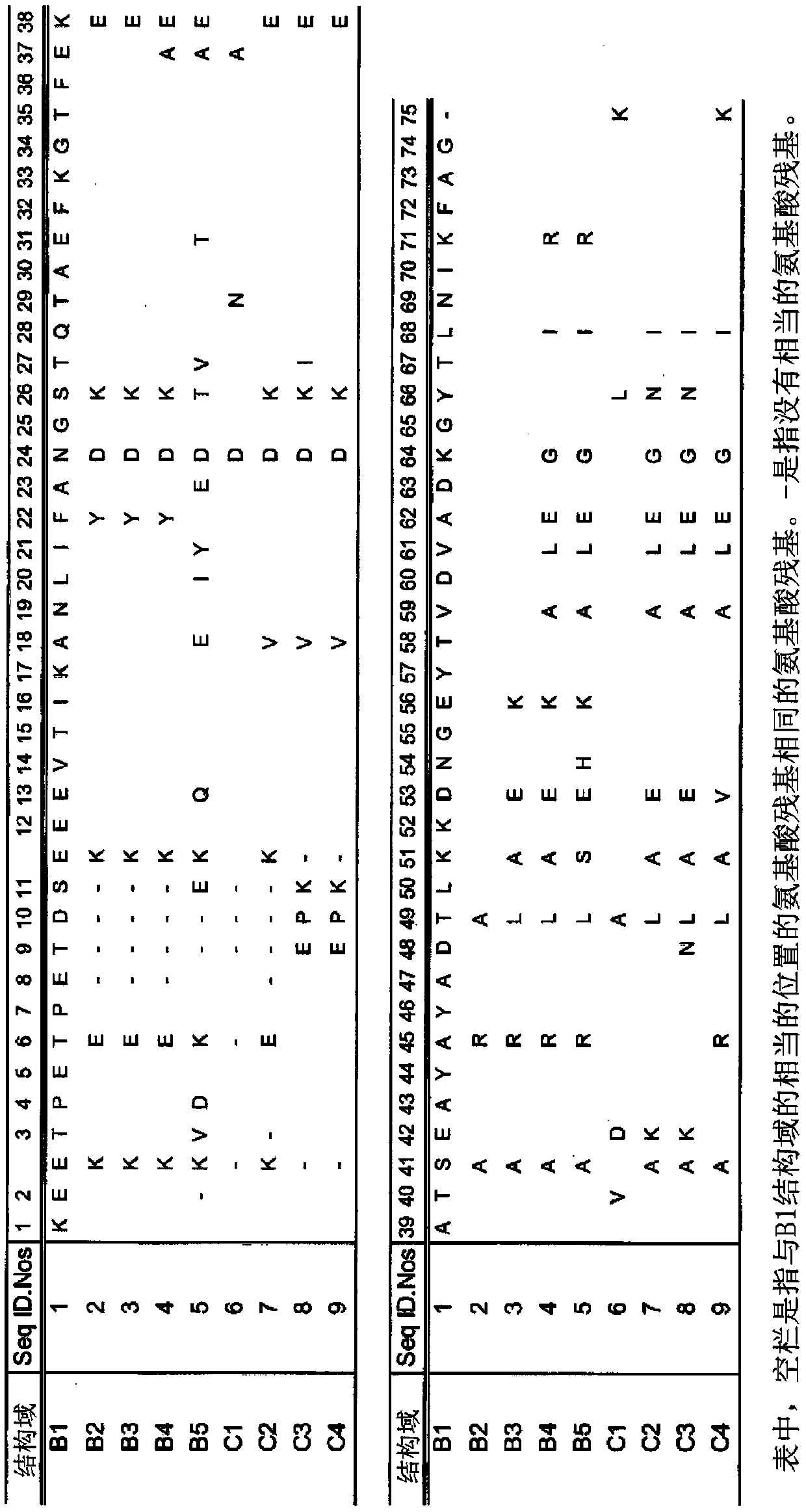
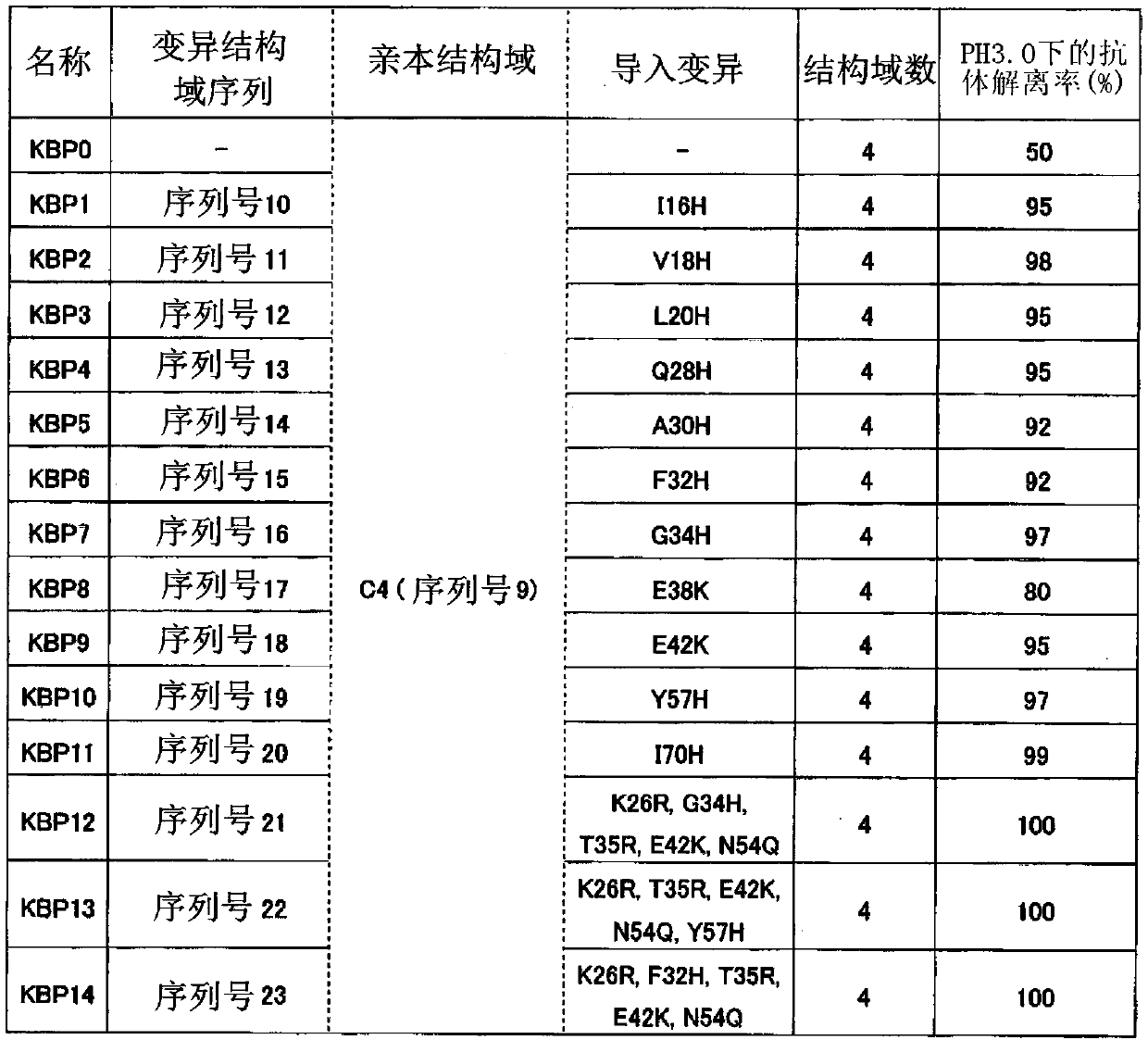
[0271] Example 1 Production of Immunoglobulin κ Chain Binding Proteins (KBP1-14)
[0272] Acquired from the artificial gene synthesis manufacturer. The gene encoding the protein comprising a plurality of immunoglobulin binding domain variants (variant domains) composed of any one of the amino acid sequences shown in SEQ ID NO: 10 to 23 was respectively encoded into pET- A plasmid derived from the 24a(+) vector. These plasmids were transformed into Escherichia coli competent cells BL21(DE3) (manufactured by NEWENGLAND BioLabs) to obtain transformed cells.
[0273]The resulting transformed cells were incubated at 37°C until the absorbance (OD600) reached about 10. Then, IPTG (manufactured by Sigma-Aldrich) was added to a final concentration of 1 mM, and further incubated at 37° C. for 4 hours to express the recombinant immunoglobulin κ chain-binding protein. Cells were recovered and disrupted in Tris buffer at pH 9.5. The recombinant immunoglobulin-binding protein was purifie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com