Composition for delivering physiologically active ingredients into blood vessel
A bioactive substance and composition technology, applied in the direction of organic active ingredients, medical preparations containing active ingredients, genetic material components, etc., can solve problems such as undeveloped delivery systems, achieve excellent embolism effects, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
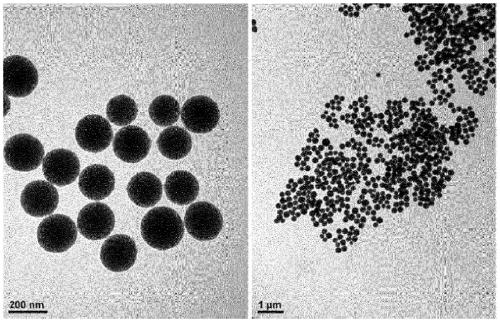
[0241] The preparation and pore expansion of the small-pore particles can be performed according to the above-mentioned procedures, and then the washing and drying procedures can be performed.
[0242] If necessary, unreacted material can be separated by separating the supernatant, eg, by centrifugation, prior to washing.
[0243] The centrifugation may be performed at, for example, 6,000 to 10,000 rpm for, for example, 3 to 60 minutes, specifically, 3 to 30 minutes, 5 to 30 minutes, etc. within the above range, but is not limited thereto.
[0244] Washing after preparing the small-pore particles may be performed in any method under the conditions within the above range, but is not limited thereto.
[0245] Washing after pore expansion can be performed under more relaxed conditions than in the illustrative embodiments described above. For example, washing may be performed three times or less, but is not limited thereto.
[0246] The surface of the particles and / or the inside...
Embodiment 1
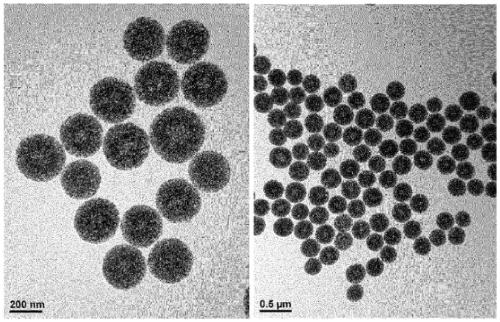
[0280] Example 1 - Preparation of Porous Silica Particles
[0281] (1) Preparation of Particle 1
[0282] 1) Preparation of Small Pore Particles
[0283] 960ml of distilled water (DW) and 810ml of MeOH were placed in a 2L round bottom flask. 7.88 g of CTAB was added to the flask, followed by the rapid addition of 4.52 ml of 1M NaOH while stirring. After introducing the homogeneous mixture under stirring for 10 minutes, 2.6 ml of TMOS were added thereto. After stirring for 6 hours for uniform mixing, the mixture was aged for 24 hours.
[0284] Then, the reaction solution was centrifuged at 25° C. and 8000 rpm for 10 minutes to remove the supernatant. During centrifugation at 25° C. and 8000 rpm for 10 minutes, the product was washed five times alternately with ethanol and distilled water.
[0285] Thereafter, the resultant was dried in an oven at 70° C. to obtain 1.5 g of powdery small-pore porous silica particles (average pore diameter: 2 nm, particle diameter: 200 nm)....
Embodiment 2
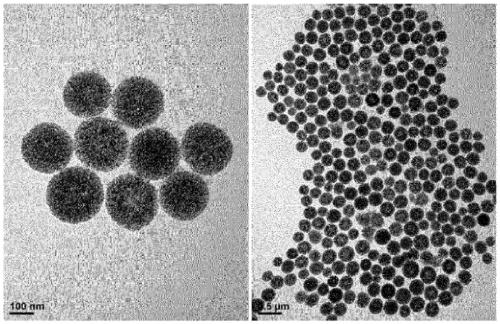
[0327] Example 2 - Surface modification of porous silica particles
[0328] (1) positive charge
[0329] 1) Amino-particles with a particle size of 300nm
[0330] The porous silica particles in Example 1-(4) were reacted with (3-aminopropyl)triethoxysilane (APTES) to be positively charged.
[0331] Specifically, 100 mg of porous silica particles were dispersed in 10 ml of toluene in a 100 ml round bottom flask by means of a bath sonicator. Then, 1 ml of APTES was added and stirred at 130° C. and 400 rpm for 12 hours.
[0332] After the reaction, the product was slowly cooled to room temperature, and then centrifuged at 8000 rpm for 10 minutes to remove the supernatant. During centrifugation at 25° C. and 8000 rpm for 10 minutes, the product was washed five times alternately with ethanol and distilled water.
[0333] Then, the washed product was dried in an oven at 70° C. to obtain powdery porous silica particles having amino groups on the surface of the particles and ins...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com