Anti-Ebola virus glycoprotein gp1 subunit monoclonal antibody 4f1 and its application
A monoclonal antibody, Ebola virus technology, applied in the field of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Antibody Preparation
[0032] 1. Blood Sample Collection
[0033] After obtaining informed consent, 5 mL of blood samples were collected 28 days after the second immunization of the recombinant Ebola vaccine clinical trial subjects for subsequent experiments.
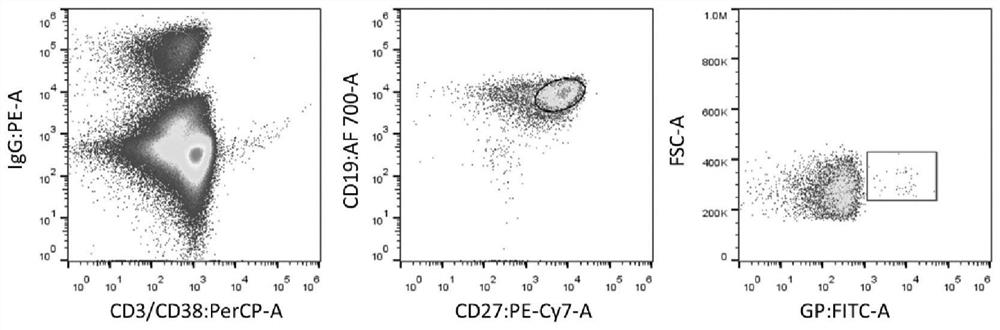
[0034] 2. FITC-labeled protein GPdM
[0035] Specific memory B cells need to be sorted by fluorescently labeled antigens. The method of FITC-labeled truncated antigen protein GPdM is as follows:
[0036] 1) Fluorescein Isothiocyanate_FITC (SIGMA, F4274) was dissolved in DMSO at a concentration of 20 mg / mL.
[0037] 2) Take 100 μL of GPdM (3.3 mg / mL), add buffer (pH9.6 carbonate buffer) to 400 μL.
[0038] 3) Add 8 μL FITC to the GPdM solution, and incubate at 4° C. in the dark for 3 hours.
[0039] 4) Use a 50kD centrifugal concentrator tube to replace the solution with PBS until the filtrate is transparent and colorless. Wrap the labeled protein in tinfoil and store at 4°C until use.
[0040] 3....
Embodiment 2
[0120] Example 2.ELISA detection of antibody binding activity
[0121] 1) The day before the experiment, 96-well enzyme-linked plates were coated with 1 μg / mL of EBOV GP, BDBV GP, SUDV GP and RESTV GP, 100 μL per well. Put the coated ELISA plate in a humid box overnight at 4°C.
[0122] 2) Wash 5 times with a plate washer on the day of the experiment.
[0123] 3) Add 100 μL of blocking solution to each well and let stand at room temperature for 1 hour.
[0124] 4) Wash the plates 5 times in a plate washer.
[0125] 5) Add 150 μL of 4F1 monoclonal antibody at a concentration of 10 μg / mL to the first well, and add 100 μL of diluent to the remaining wells. Aspirate 50 μL from the first well and add to the second well, and so on, dilute the solution in a 1:3 gradient to a final volume of 100 μL in each well. Let stand at room temperature for 1 hour.
[0126] 6) Wash the plates 5 times in a plate washer.
[0127] 7) Dilute the HPR-labeled goat anti-human IgG secondary antibod...
Embodiment 3
[0132] Embodiment 3. Pseudovirus neutralization experiment evaluates 4F1 neutralization activity
[0133] The EBOV pseudovirus packaged with the HIV backbone was used to evaluate the neutralizing activity of 4F1 in vitro, as follows:
[0134] 1) Dilute the 4F1 monoclonal antibody with DMEM medium, add 75 μL of antibody diluent with a concentration of 100 μg / mL to the first well of a 96-well cell culture plate, and add 50 μL of DMEM medium to the remaining wells.
[0135] 2) Pipette 25 μL of liquid from the first well into the second well, mix well, and so on, dilute at a ratio of 1:3, and the final volume of each well is 50 μL.
[0136] 3) Dilute the pseudovirus 1:5 with DMEM medium (control the fluorescein reading in the control well between 20,000 and 100,000), add to each antibody well, 50 μL per well. Mix well and incubate at 37°C for 1h.
[0137] 4) 293T cell count, 2×10 5 cells / mL, add 100 μL to each well.
[0138] 5) Put the 96-well cell culture plate into a 37°C th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com