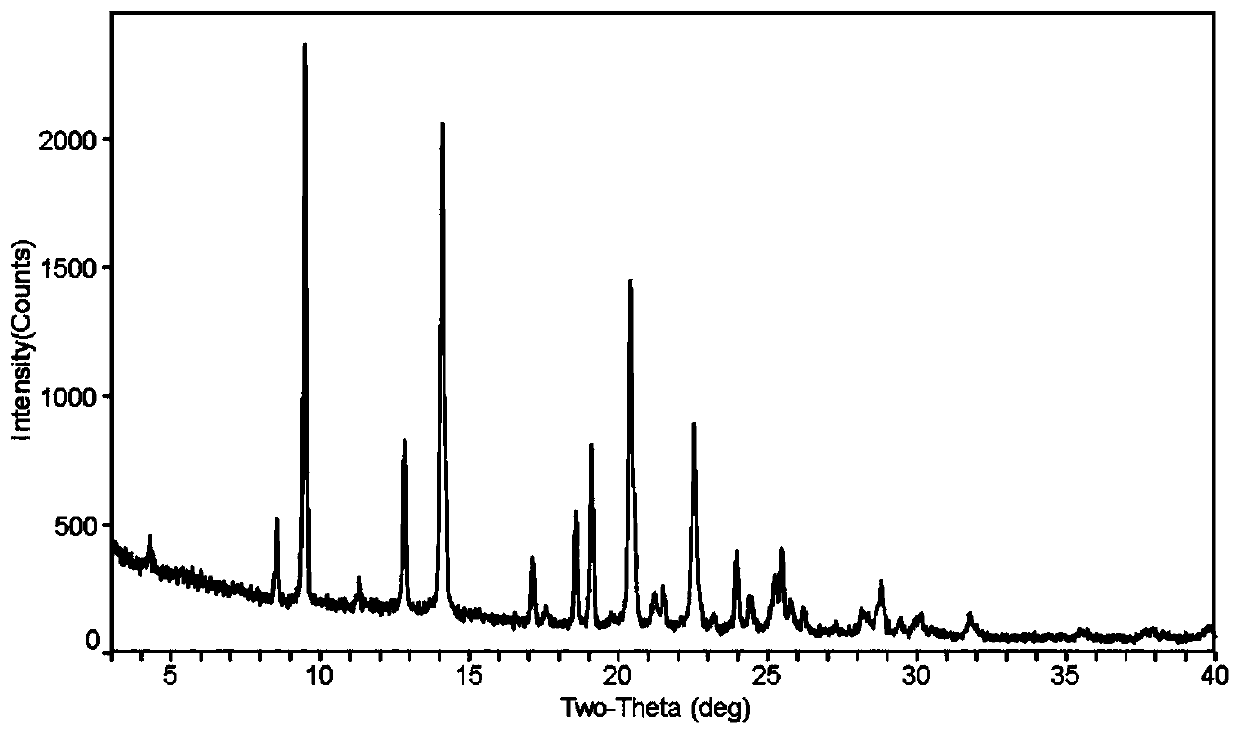
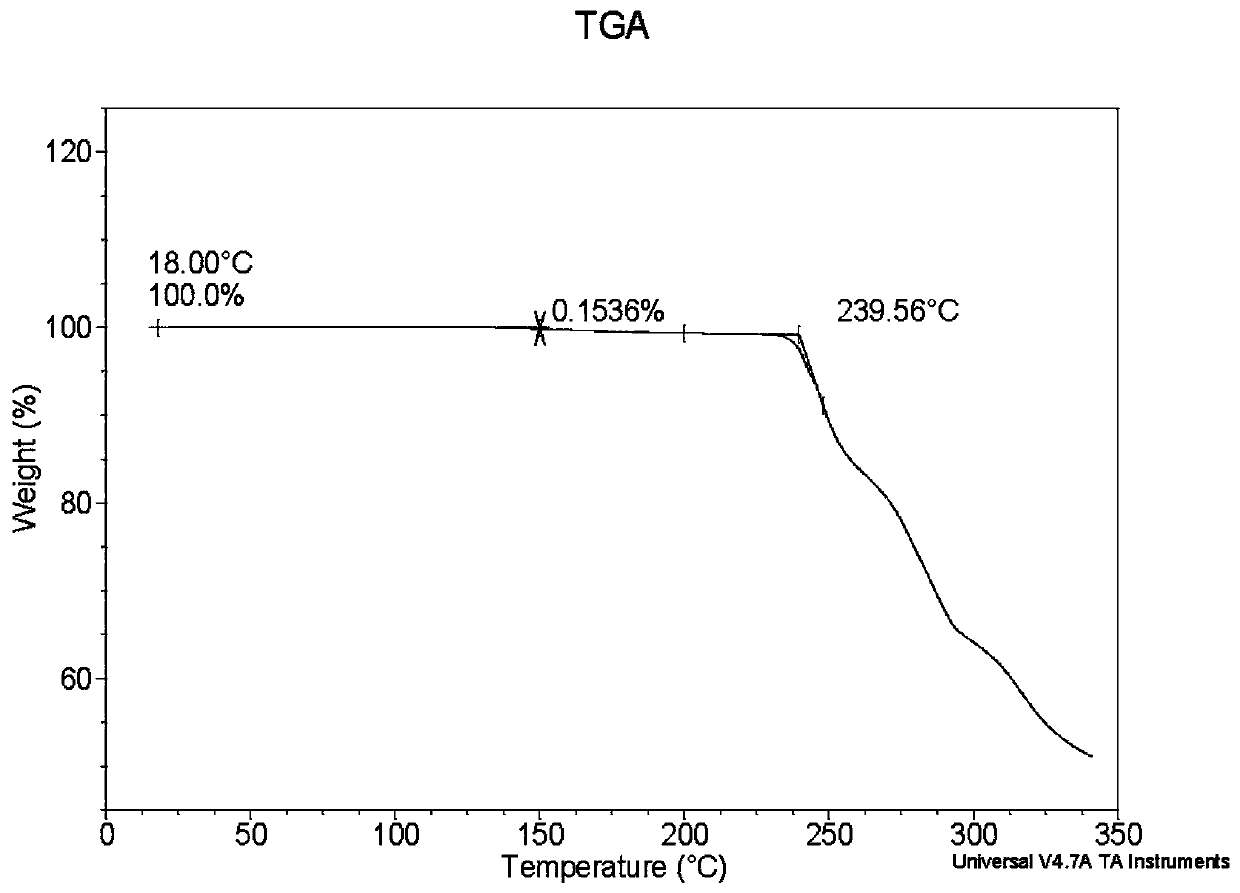
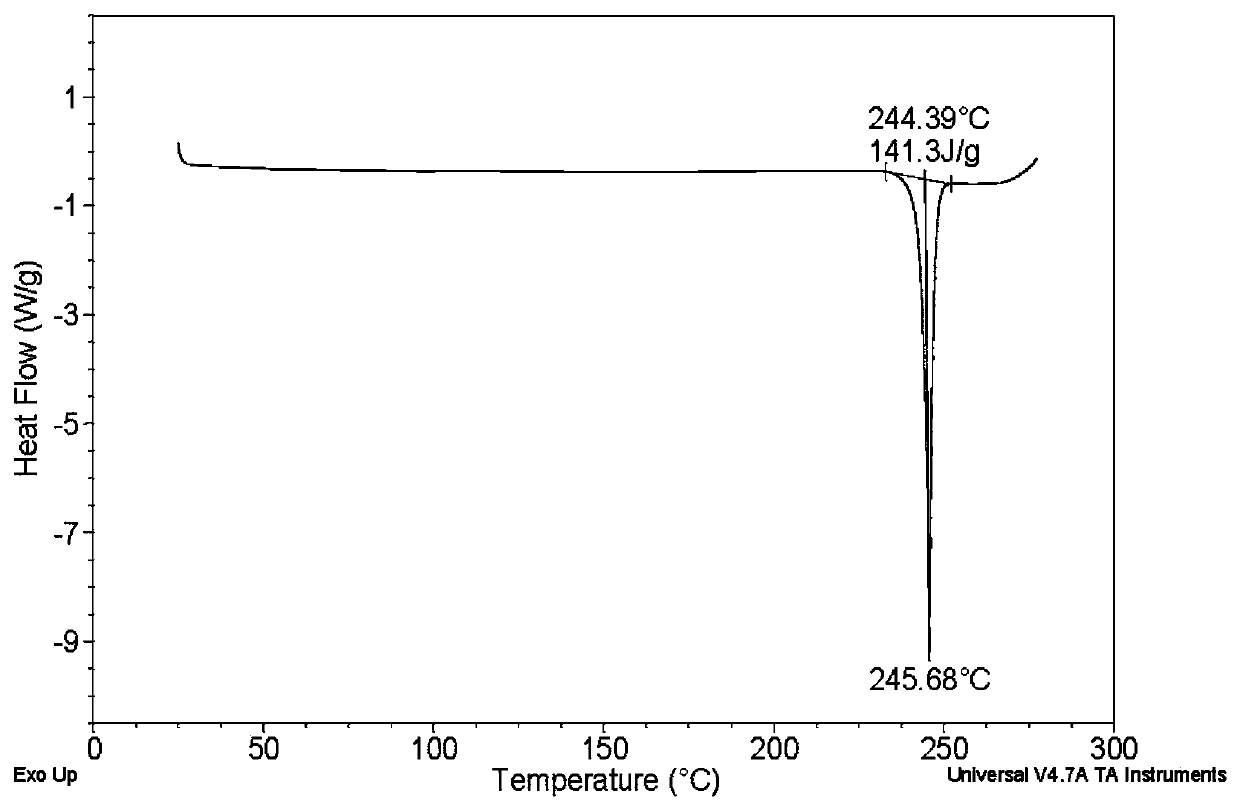
Crystal form of quinazoline compound and preparation method thereof
A technology for quinazolines and compounds, which is applied in the field of crystal forms of quinazolines and their preparation, and can solve problems such as excessive cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] This embodiment is the preparation method of quinazoline compound crystal form A described in the present invention, comprising the following steps:
[0123] (1) The crude product 3.00g of quinazoline compound is washed 2 times with purified water (4.65g), ethanol (1.90g), after controlling vacuum-0.08MPa temperature 20 ℃ of drying 2 hours in vacuum oven, then heat up Dry at 50°C for 20 hours, take out the solid, then add 38.0g of dimethyl sulfoxide (DMSO) to 40°C and stir to dissolve, filter, add purified water (70g) and stir, and then filter;
[0124] (2) The quinazoline compounds that have completed step (1) are washed sequentially with 7.0 g of purified water and 3.8 g of ethanol, then heated to 78°C with 37.0g of ethanol for beating under reflux for 3 hours, cooled to 20°C for filtration, and then After washing with 3.70g of ethanol, add 27g of absolute ethanol, then add 7.80g of dimethyl sulfoxide (DMSO), heat at 78°C, continue stirring for 3h, drop to 20°C and fi...
Embodiment 2
[0127] This embodiment is the preparation method of quinazoline compound crystal form A described in the present invention, comprising the following steps:
[0128] (1) 3.00 g of the crude product of quinazoline compounds are washed twice with purified water (4.65 g), ethanol (1.90 g), and after drying for 2 hours at a temperature of 30° C. of vacuum-0.10 MPa in a vacuum oven, the temperature is raised Dry at 55°C for 20 hours, take out the solid, then add 38.0g of dimethyl sulfoxide (DMSO) to 45°C and stir to dissolve, filter, add purified water (70g) and stir, and then filter;
[0129] (2) The quinazoline compounds that have completed step (1) are washed sequentially with 7.0 g of purified water and 3.8 g of ethanol, then heated to 70°C with 37.0g of ethanol to reflux for beating for 2 hours, cooled to 20°C for filtration, and then After washing with 3.70g of ethanol, add 27g of absolute ethanol, then add 7.80g of dimethyl sulfoxide (DMSO), heat at 83°C, continue to stir for...
Embodiment 3
[0132] This embodiment is the preparation method of quinazoline compound crystal form A described in the present invention, comprising the following steps:
[0133] (1) The crude product 3.00g of quinazoline compound is washed 2 times with purified water (4.65g), ethanol (1.90g), after controlling vacuum-0.09MPa temperature 20 ± 10 ℃ of drying 2 hours in vacuum oven, Then raise the temperature to 45°C and dry for 20 hours, take out the solid, then add 38.0 g of dimethyl sulfoxide (DMSO) and stir to dissolve at 35°C, filter, add purified water (70 g) and stir, and then filter;
[0134] (2) The quinazoline compounds that have completed step (1) are washed sequentially with 7.0 g of purified water and 3.8 g of ethanol, then heated to 80° C. for reflux with 37.0 g of ethanol for beating for 5 hours, cooled to 20° C., filtered, and then After washing with 3.70g of ethanol, add 27g of absolute ethanol, then add 7.80g of dimethyl sulfoxide (DMSO), heat at 72°C, continue stirring for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com