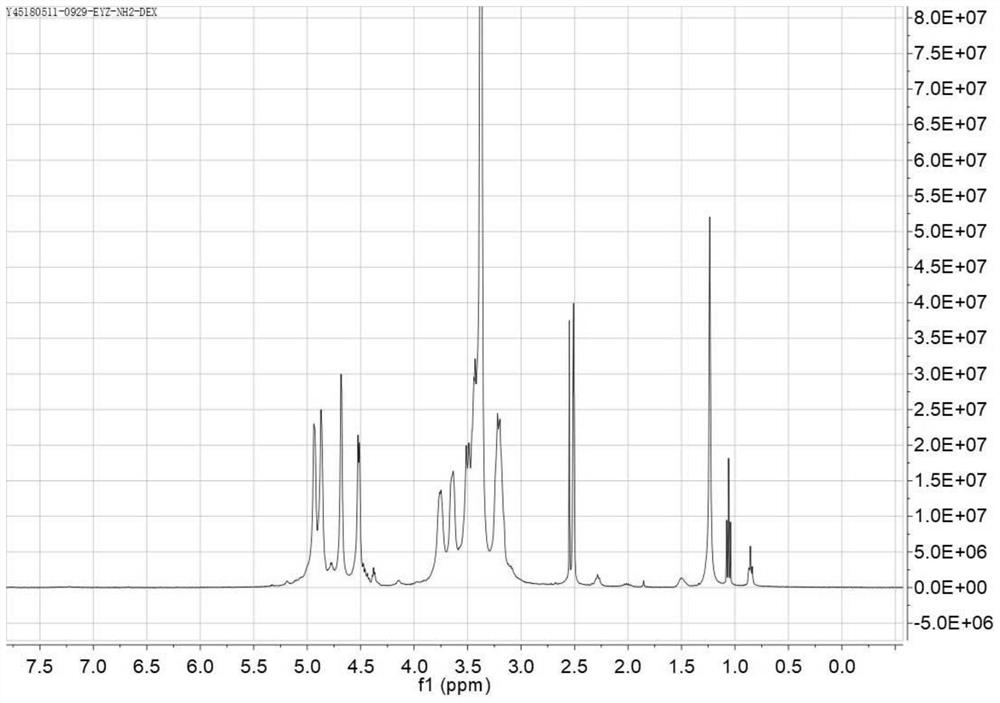
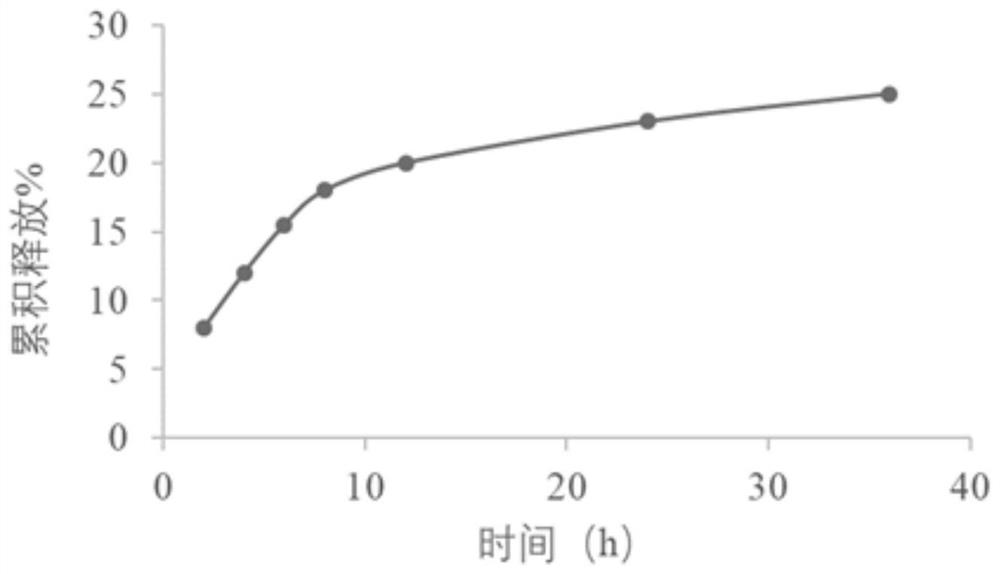
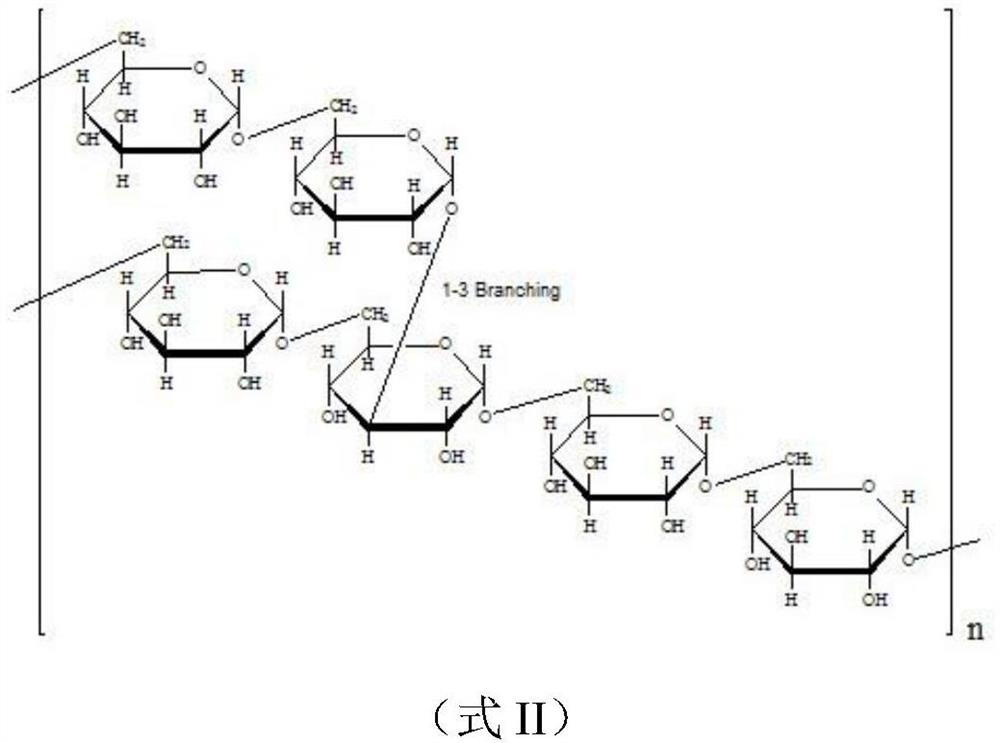
Dextran derivative and its preparation method and additives for preparation of medicament
A technology of derivatives and dextran, which is applied in the field of dextran derivatives, additives for the preparation of pharmaceuticals, and amphiphilic dextran derivatives, can solve problems such as difficult synthesis, strong hemolysis, and uncertain grafting positions , to achieve good surface activity, low hemolysis, and clear grafting position
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1. Dextran derivatives of formula I-1-1 and formula I-1-2
[0071] In this embodiment, a dextran derivative is provided, which has the structure shown in formula I-1 or formula I-2.
[0072]
[0073] The preparation method of the above-mentioned dextran derivatives is to mix the amino derivatives of diglyceride and dextran in the solution according to the molar ratio of 1:1-10:1, and generate the target product after reductive amination reaction. Wherein, the said diglycerides contain fatty acid chains with 10-20 carbon atoms. In this embodiment, the preparation method includes the following steps:
[0074] a. Steps for preparing amino derivatives of diglycerides
[0075] making the di-fatty acid glyceride react with excess compound 1 at room temperature under the action of an activator to obtain an amino derivative of the di-fatty acid glyceride;
[0076] In this step, the di-fatty acid glyceride is at least one of 1,2-difatty acid glyceride or 1,3-dif...
Embodiment 2
[0085] Example 2. Specific Dextran Derivatives
[0086] In this example, dextran derivatives with different average molecular weights of dextran and different fatty acid glycerides were used to prepare dextran derivatives, and the obtained dextran derivatives were respectively labeled, as shown in Table 1 below.
[0087] Table 1. Dextran and corresponding dextran derivatives with different average molecular weights
[0088]
[0089]
[0090] The above compounds have the following general formula
[0091]
[0092] Wherein, the value range of n and o is 8-20, preferably 14-16; and, n and o may be the same or different values.
[0093] Those skilled in the art can understand that the glyceryl distearate described in Table 1 may be glyceryl distearate or glyceryl 1,3-distearate or a mixture of both.
[0094] In this example, according to the dextran and diglycerides listed in Table 1, the dextran derivatives were prepared according to the following specific steps to obt...
Embodiment 3
[0098] Embodiment 3. Comparative Examples
[0099] In this example, octadecylamine is used as a hydrophobic group to bond to the reducing end of dextran with an average molecular weight of 10,000 to obtain a dextran derivative DEX10K-OCA, which is A one-tailed dextran derivative.
[0100] The specific preparation method of the dextran derivative DEX10K-OCA is as follows.
[0101] Take 0.1mmol of 1.0g of dextran and put it in a dry flask, add 20ml of anhydrous DMSO, heat and stir at 60°C until dissolved; add 0.5mmol of 135mg of octadecylamine, heat and stir at 75°C until dissolved, add 0.3ml of glacial acetic acid , heated and stirred for 2 hours. A DMSO solution (2 ml) containing 0.45 mmol of sodium triacetoxyborohydride was added, and the reaction was continued to stir at room temperature for 2 hours.
[0102] Repeatedly filter the reaction solution until it becomes clear, add the filtrate to about 5 times the amount of ethanol to cause precipitation, filter, take the filt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com