Novel application of Plerixafor in preparation of drugs for treating or preventing diseases related to GSDMD protein
A technology of plerixafor and disease, which is applied in the field of medicine, can solve the problems such as the use of infectious inflammatory diseases that have not been reported, and achieve the effects of reducing clinical scores and morbidity, reducing secretion, and increasing the number of monocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
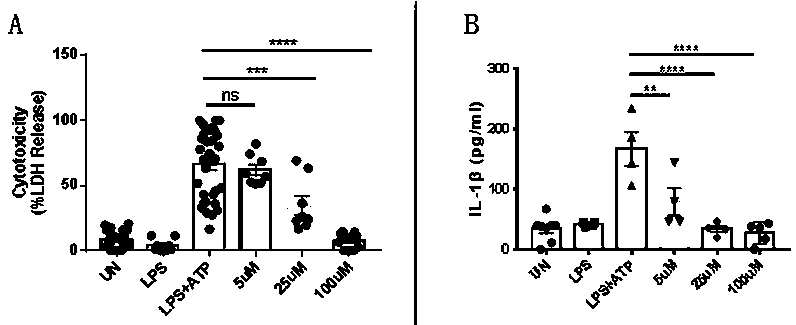
[0043] Example 1: Plerixafor can reduce the cytotoxicity and IL-1β secretion of immortalized mouse bone marrow-derived macrophages
[0044] 1.1) Cell culture
[0045] Immortalized mouse bone marrow-derived macrophages (iBMDMs) were cultured in DMEM medium containing 10% FBS
[0046] 1.2) iBMDMs grouping and processing
[0047] On the first day, the cells were seeded on a 96-well plate, 5*10 per well 4 cells;
[0048] On the second day, the iBMDMs in the 96-well plate were randomly divided into UN group (control group), LPS group, LPS+ATP group, plerixafor 5uM group, plerixafor 25uM group and plerixafor 100uM group, each The iBMDMs in each well were centrifuged to remove the supernatant, and then the DMEM medium containing 10% FBS without LPS was added to the UN group, and the other five groups were added with LPS (100ng / ml) of DMEM medium containing 10% FBS in 5% CO 2 After culturing in the incubator at 37°C for three hours, the iBMDMs of each group were treated as follo...
Embodiment 2
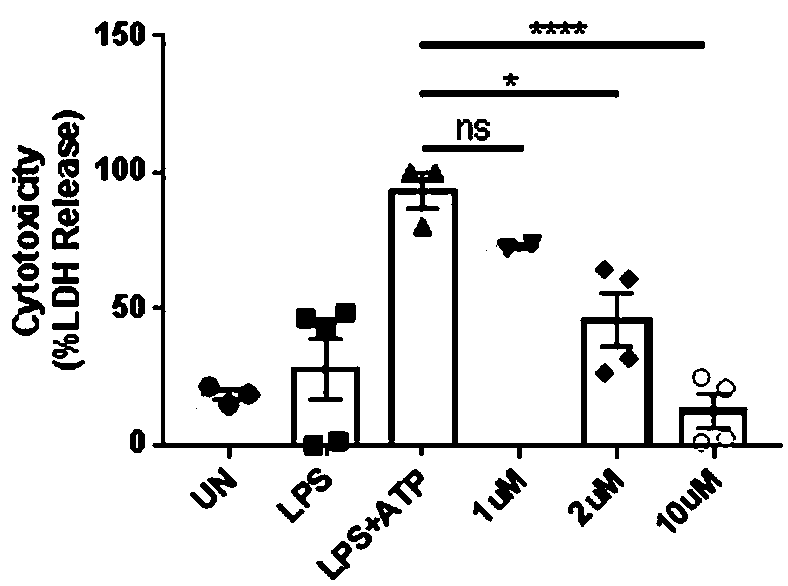
[0059] Example 2: Plerixafor can reduce the cytotoxicity of human mononuclear macrophage cell line
[0060] 2.1) Cell culture
[0061] A human mononuclear macrophage cell line (THP-1) was cultured in RPMI medium (Gibco) containing 10% FBS.
[0062] 2.2) THP-1 grouping and processing
[0063] On the first day, the cells were divided into 96-well plates, 5*10 per well 4 cells
[0064] On the second day, THP-1 in 96-well plate was randomly divided into UN group (control group), LPS group, LPS+ATP group, plerixafor 5uM group, plerixafor 25uM group and plerixafor 100uM group , 6 replicate wells per group, 5*10 per well 4 For each well, add phorbol 12-myristate-13-acetate (phorbol-12-myristate-13-acetate, PMA) to a final concentration of PMA of 100 nM to stimulate cell differentiation and adhesion in each well. After 4 hours of differentiation treatment, the supernatant was removed, then the UN group was added the RPMI medium containing 10% FBS without LPS, and the other five g...
Embodiment 3
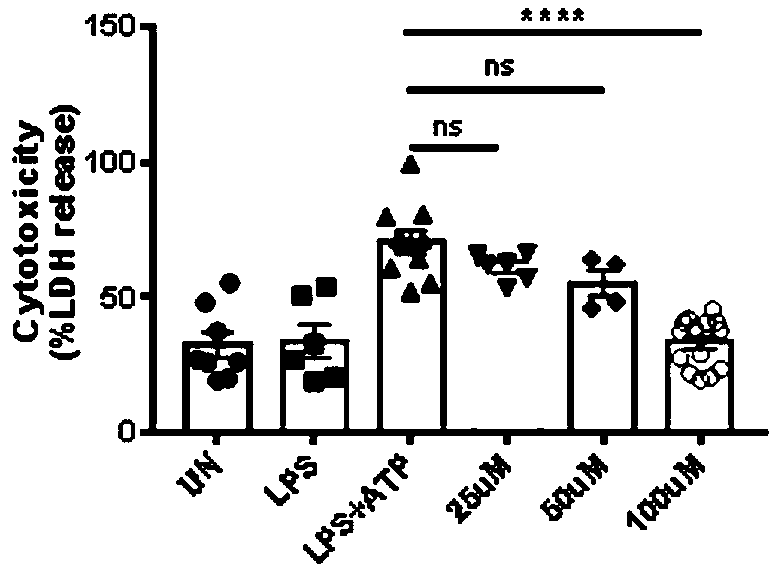
[0075] Example 3: Plerixafor can reduce the cytotoxicity of mouse bone marrow-derived macrophages
[0076] 3.1) Cell culture
[0077] Use DMEM medium containing 20% L929 cell (mouse fibroblast) culture supernatant to cultivate mouse bone marrow-differentiated macrophages (BMDMs). L929 cell culture supernatant was cultured and differentiated in DMEM medium for 4 days; on the fifth day, BMDMs cells were seeded on 96-well plates, 5*10 per well 4 cells, and the culture medium was replaced with DMEM medium containing 10% FBS to continue culturing;
[0078] 3.2) BMDMs grouping and processing
[0079] Step 3.1) On the sixth day of cell culture, the BMDMs in the 96-well plate in 3.1) were randomly divided into UN group, LPS group, LPS+ATP group, plerixafor 1uM group, plerixafor 2uM group and plerixafor 10uM group, 6 replicate wells in each group, 5*10 in each well 4 Remove the supernatant from the BMDMs in each well, then add DMEM medium containing 10% FBS without LPS to the UN ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com