Crosslinkable electroactive fluorinated polymers
A technology of copolymers and compositions, applied in the direction of coatings, etc., can solve the problems of deterioration of electroactive properties and complex preparation of polymer films
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1 - Manufacture of modified polymers according to the invention
[0178] The starting material used was a P(VDF-TrFE-CTFE) terpolymer. The terpolymer comprises 61.8 mol % of units derived from VDF, 30.4 mol % of units derived from TrFE and 7.8 mol % of units derived from CTFE.
[0179] 1.2 g of terpolymer powder was dissolved in 50 ml of dimethylformamide. Then 39 mg (0.5 molar equivalent, relative to the number of moles of CTFE) of NaN 3 Add to reaction mixture. The reaction was maintained at 55°C for 12 hours. The product was recovered after precipitation from deionized water. It was then filtered and dried under vacuum at 40° C. for 24 hours.
[0180] Then with 0.1 molar equivalent or 10 molar equivalent of NaN 3 Repeat the experiment relative to moles of CTFE.
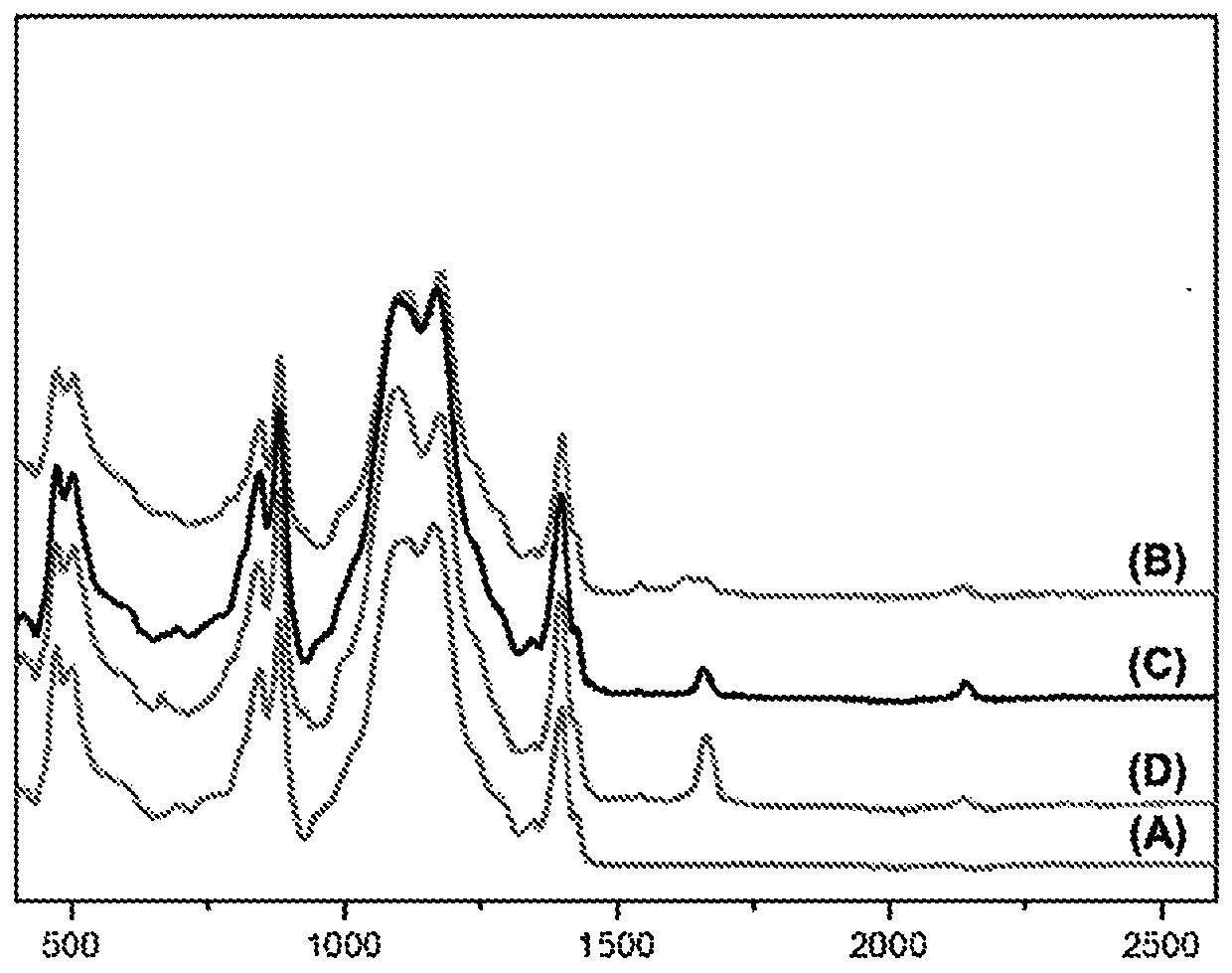
[0181] Infrared spectra of various polymers were obtained directly on polymer films using a Fourier Transform Infrared (FTIR) spectrometer in ATR (reflection) mode:
[0182] The results are s...
Embodiment 2
[0188] Example 2 - Fabrication of a film according to the invention with UV irradiation
[0189] The 7 mass % formulation in butan-2-one was obtained by mixing an unmodified P(VDF-TrFE-CTFE) terpolymer comprising 7.8 mol % of CTFE-derived units in a 50 / 50 mixed (by mass) with an additional P(VDF-TrFE-CTFE) terpolymer initially comprising 12.7 mol % of units derived from CTFE, and in the same manner as in Example 1 with 0.5 equivalents of NaN 3 modified.
[0190] From the formulations prepared above, 250 nm films were fabricated on silicon substrates on a spin coater. The resulting film was subsequently dried at 60° C. for 5 minutes.
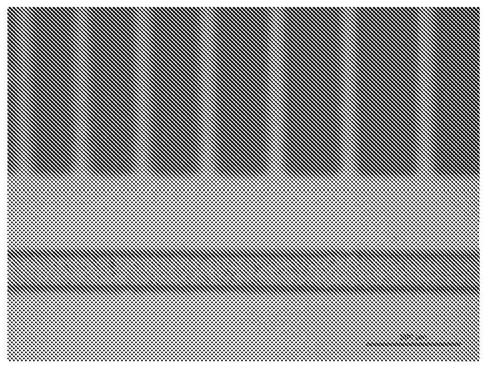
[0191] The film was crosslinked according to a predetermined pattern by UV irradiation (mainly at a wavelength of 300 to 400 nm) at an applied dose of 20 J / cm 2 . The membrane was then developed by rinsing in cyclopentanone for 1 minute at ambient temperature.
[0192] available at figure 2 See the resulting pattern in the picture. The d...
Embodiment 3
[0199] Example 3 - Manufacture of a membrane according to the invention by heat treatment
[0200] Using the modified polymer of Example 1 (with 0.5 molar equivalents of NaN 3 obtained), and to fabricate membranes from this polymer.
[0201] The film has a thickness of 2 μm. It was fabricated on a spin coater and dried at 60 °C for 5 min.
[0202] Infrared spectra of the films were measured before and after crosslinking. Heat crosslinking at 125°C for 20 minutes.
[0203] result in Figure 5 visible in the figure. The upper spectrum is the spectrum of the film before crosslinking and the lower spectrum is the spectrum of the film after crosslinking. The disappearing band was observed at 2150cm -1 The band at (which is characteristic of the azide functional group) and at 1710cm -1 A band at (which is characteristic of an intrachain C=C double bond).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com