Formula and process of all-vanadium redox flow battery electrolyte
An all-vanadium redox flow battery and electrolyte technology, which is applied in the field of all-vanadium redox flow battery electrolyte, can solve the problems of increasing the conductive resistance of the vanadium electrolyte, increasing the internal resistance of the electrolyte, and reducing the charging and discharging efficiency, and achieves the procurement cost. Reduces, improves performance, extends service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
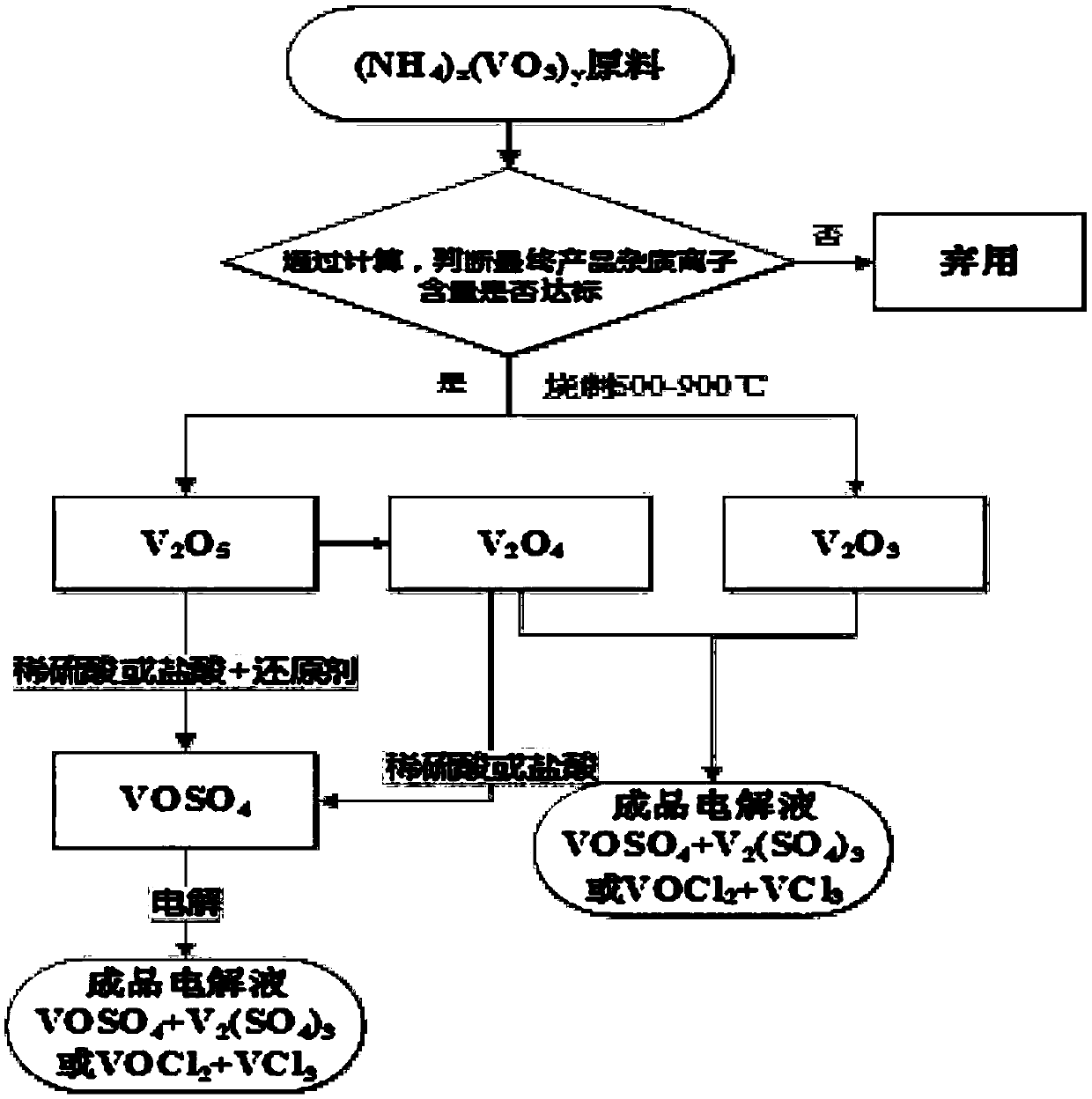
Image
Examples
Embodiment 5
[0049] Example 5 is compared with Comparative Example 2; wherein the experimental conditions of Example 5 are the same as those of Comparative Example 2; the scale of the stack, the charge-discharge mode, the operating time and other conditions are all the same;
Embodiment 6
[0050] Example 6 is compared with Comparative Example 3; wherein the experimental conditions of Example 6 are the same as those of Comparative Example 3; the scale of the stack, the charge-discharge mode, the operating time and other conditions are all the same;
[0051] The single elements in the VIII subgroup elements in the examples are Ni, Co, and Fe.
Embodiment 1
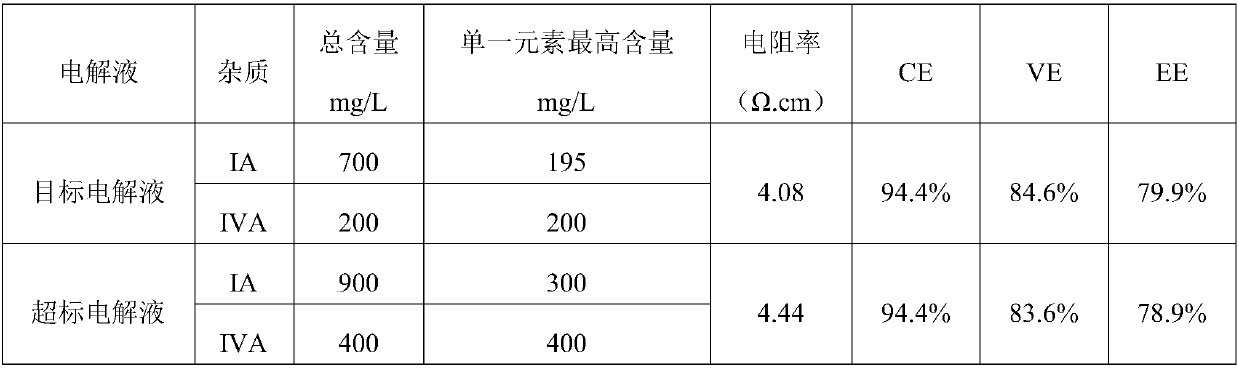
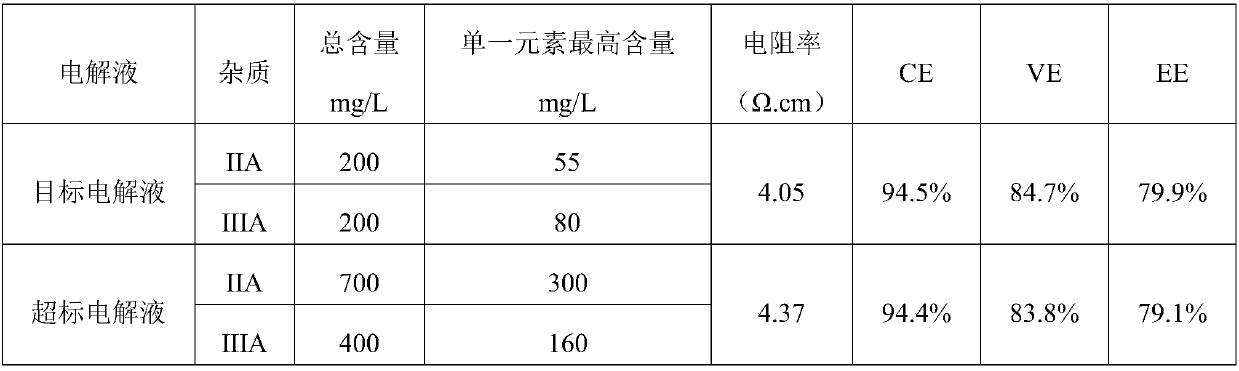
[0071] The vanadium electrolyte was prepared and operated according to the sample content in the following table, and the results were shown in the following table:
[0072]
[0073] The above data and operating results show that when the IA main group and IVA main group elements are appropriately relaxed, the target electrolyte has no effect on the internal resistance of the electrolyte, and the stack efficiency is no different from that of the high-purity electrolyte stack. However, when the impurity elements exceed the standard, the efficiency of the stack decreases to a certain extent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com