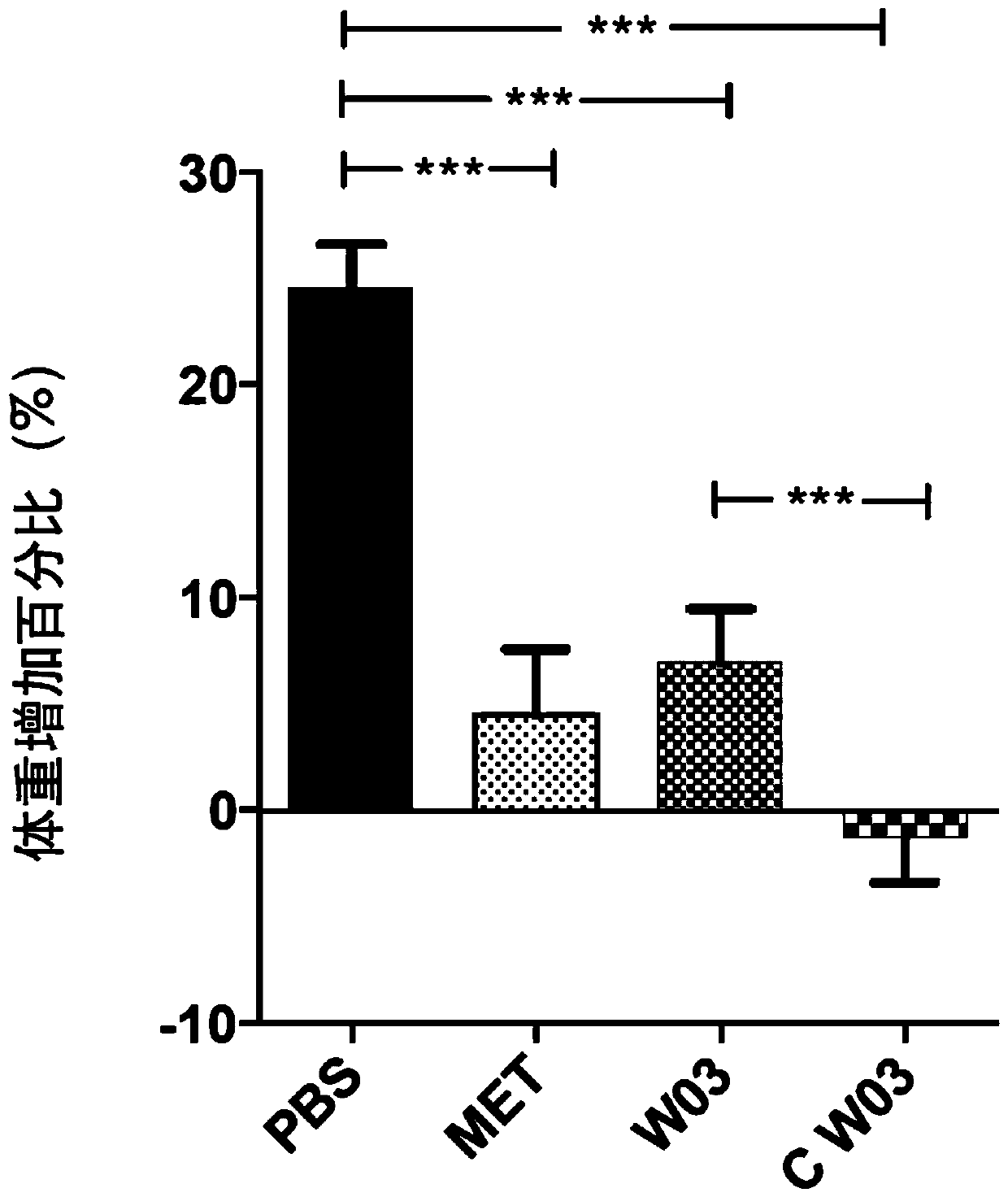
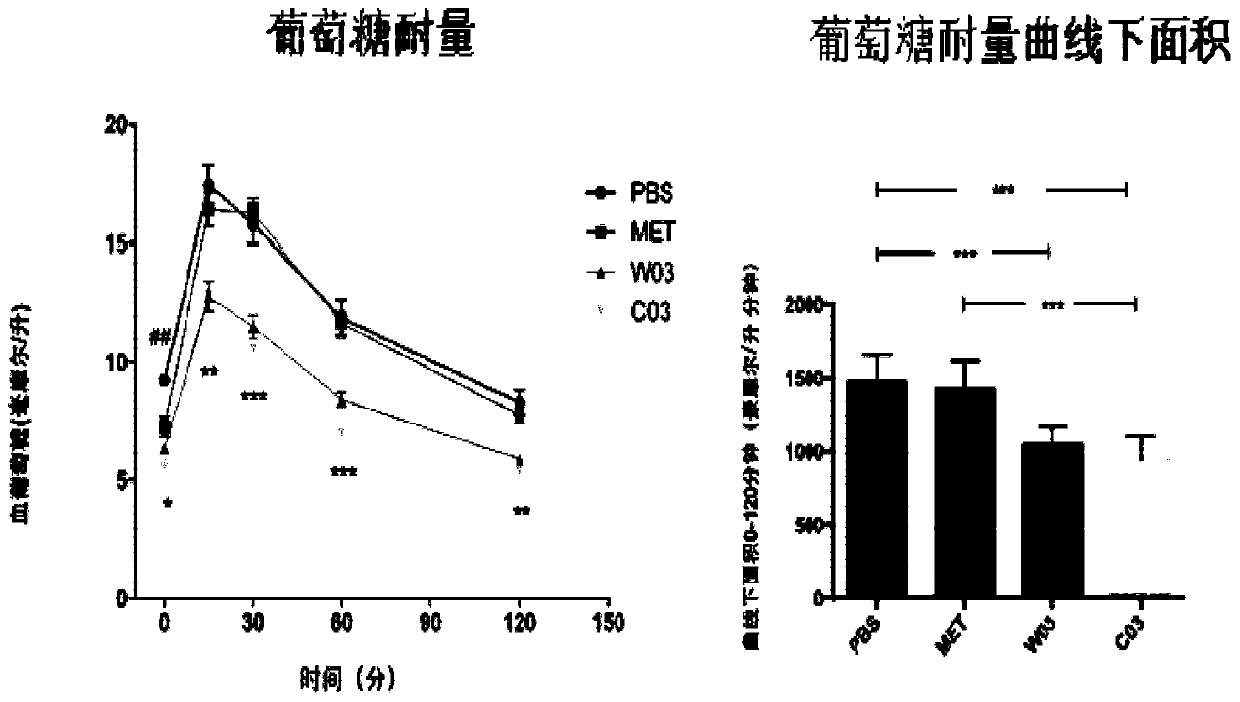
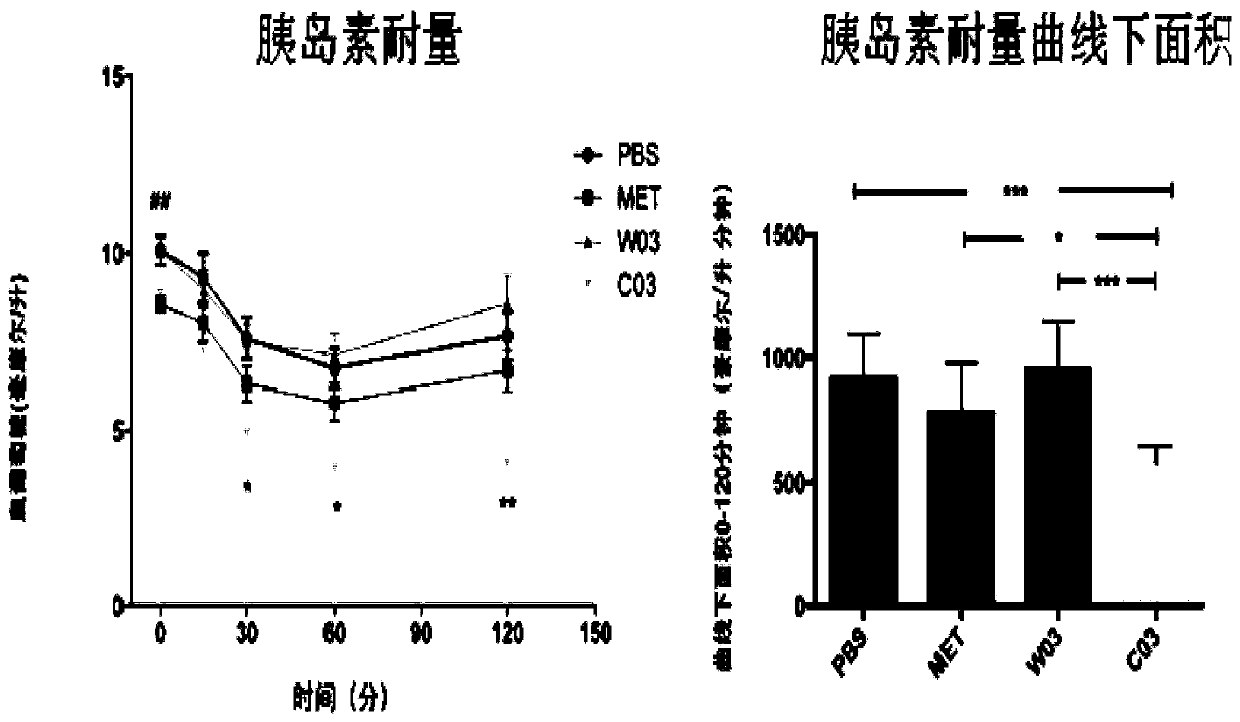
Akkermansia muciniphila composition
A composition and drug technology, applied in the field of Akkermansia composition, can solve the problems of high risk of blood sugar excursion and superposition of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Wherein, the preparation method of the Akkermansia freeze-dried powder preferably comprises the following steps:
[0047] The centrifuged cells of Akkermansia are evenly mixed with freeze-drying protective agent and water, and then freeze-dried.
[0048] Wherein, the centrifuged cells of Akkermansia are preferably obtained according to the following method: centrifuge the fermentation liquid of Akkermansia and collect the cells. The centrifugation is a conventional operation in the field, for example, centrifugation at 8000 rpm for 20 minutes.
[0049] Wherein, based on the mass of the pharmaceutical composition, the viable count of Akkermansia in the Akkermansia freeze-dried powder is preferably 10 6 -10 14 CFU / g, more preferably 10 10 CFU / g.
[0050] Wherein, the raw material of the lyoprotectant is a conventional lyoprotectant raw material in the art. For example, in a preferred embodiment of the present application, the lyoprotectant is a mixture of sodium glut...
Embodiment 1
[0081] Embodiment 1: Akkermansia metformin compound tablet preparation
[0082] (1) Preparation of W03 freeze-dried powder
[0083] ① Freeze-drying protective agent: sodium glutamate, mannitol, trehalose, milk powder.
[0084] ② After the fermentation of W03 bacteria, the fermentation liquid was centrifuged at 8000rpm for 20min to collect the bacteria.
[0085] ③ Akkermansia centrifugation obtained thalline is mixed with freeze-drying protective agent and water, so that the mass percentages of sodium glutamate, mannitol, trehalose, and milk powder in the solution before freeze-drying are respectively 2%, 2%, 4% and 8%, freeze-dried to obtain freeze-dried bacterial powder, the number of live bacteria of the Akkermansia strain whose preservation number is CGMCC No.14764 in the bacterial powder is 10 10 CFU / g. The obtained W03 bacteria powder is pulverized and stored in a turnover barrel sealed for future use.
[0086] (2) Preparation of Metformin Granules
[0087] 10kg of m...
Embodiment 2
[0091] Example 2: Preparation of Akkermansia metformin compound enteric-coated capsules
[0092] (1) Preparation of W03 freeze-dried powder
[0093] ① Freeze-drying protective agent: sodium glutamate, mannitol, trehalose, milk powder.
[0094] ②After the fermentation of W03 bacteria, the fermentation liquid was centrifuged at 8000rpm for 20min, and the bacteria were collected.
[0095] ③ Akkermansia centrifugation obtained thalline is mixed with freeze-drying protective agent and water, so that the mass percentages of sodium glutamate, mannitol, trehalose, and milk powder in the solution before freeze-drying are respectively 2%, 2%, 4% and 8%, freeze-dried to obtain freeze-dried bacterial powder, the number of live bacteria of the Akkermansia strain whose preservation number is CGMCC No.14764 in the bacterial powder is 10 10 CFU / g. The obtained W03 bacteria powder is pulverized and stored in a turnover barrel sealed for future use.
[0096] (2) Preparation of Metformin Gra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com