Apixaban co-micronized product
A technology of co-micronization and apixaban, which is applied in the field of medicine, can solve the problems of poor reproducibility, poor compressibility of excipients, and difficult particle control, so as to improve bioavailability, increase dissolution rate, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
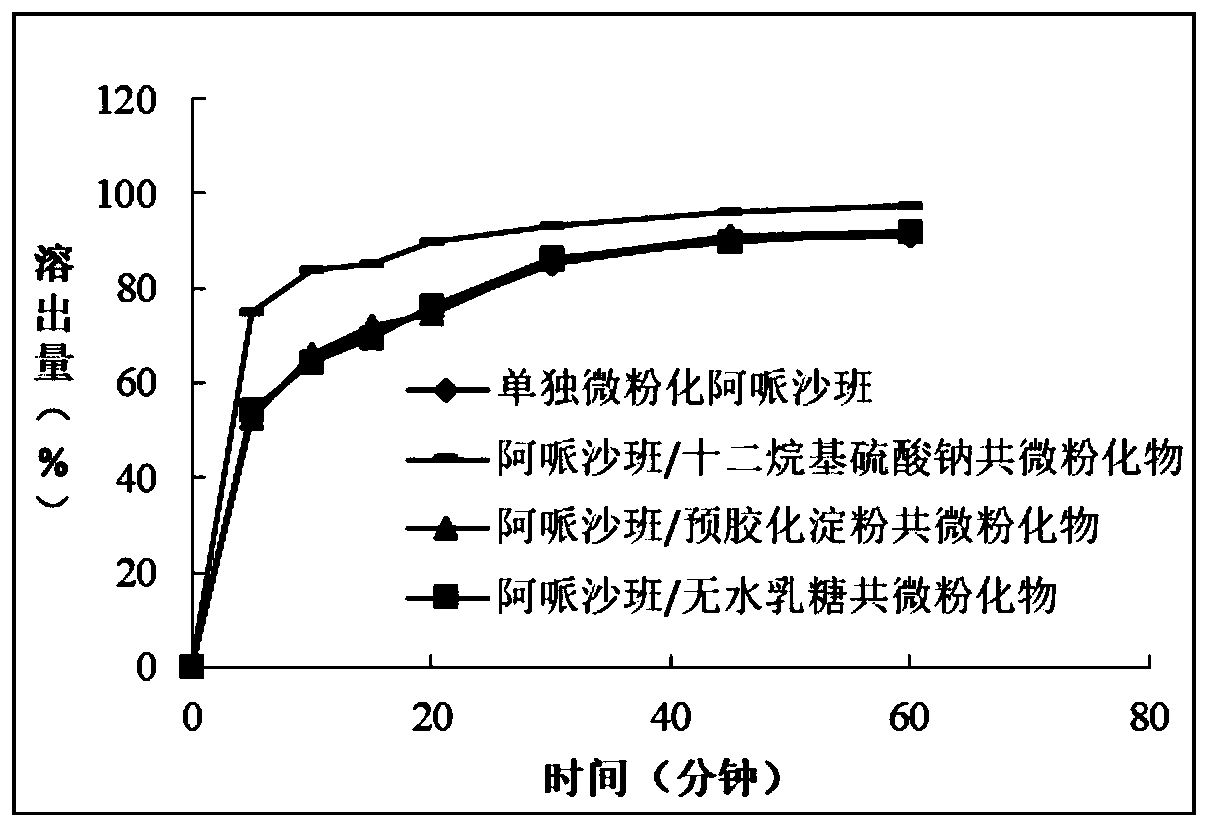
[0047] Example 1: Screening of excipients for co-micronization of apixaban
[0048] 1. Materials and methods
[0049] Apixaban was mixed with the co-micronized excipients to be tested in the desired weight ratios in a hopper mixer until a homogeneous mixture was obtained. Subsequently, the obtained mixture was micronized in a jet mill.
[0050] In Vitro Dissolution of Comicronized Apixaban
[0051] For each co-micronized product obtained, a tablet containing an amount corresponding to 2.5 mg of apixaban was prepared.
[0052] The dissolution determination method adopts the second method in the dissolution determination method of the Chinese Pharmacopoeia 2015 edition, selects 0.05mol / L sodium phosphate solution (adjusting the pH value to 6.8 with phosphoric acid) as the dissolution medium with distinguishing power, paddle method 75 rpm, The temperature is 37±0.5°C, and it is determined by a Lugen dissolution apparatus.
[0053] The sampling time is 5, 10, 15, 20, 30, 45,...
Embodiment 2
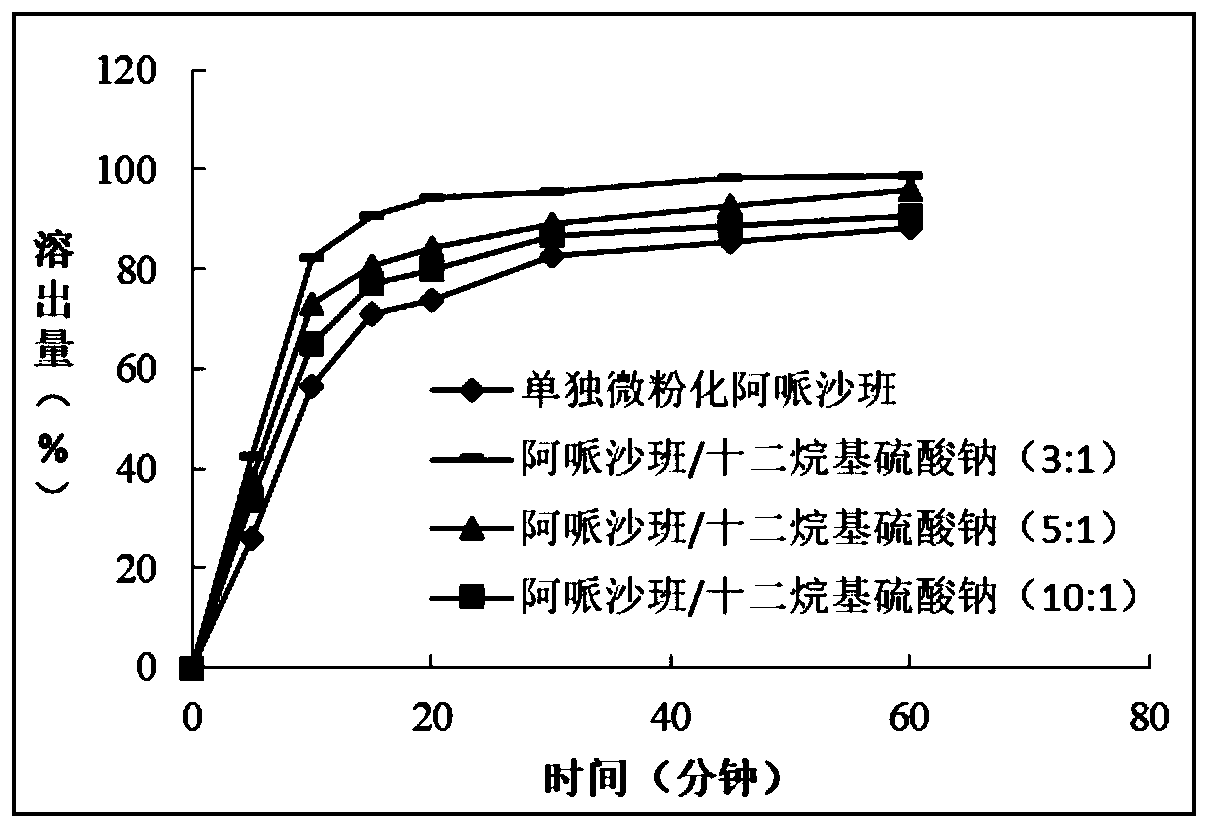
[0061] Example 2 Effect of Apixaban / Sodium Lauryl Sulfate Weight Ratio on the In Vitro Dissolution of Apixaban
[0062] 1. Materials and methods
[0063] Various apixaban / sodium lauryl sulfate co-micronized products were prepared according to the co-micronization method described above in order to study the effect of apixaban / sodium lauryl sulfate weight ratio on the in vitro effect of apixaban effect on the dissolution rate.
[0064] 2. Results
[0065] Table 2 below and figure 2 The dissolution results obtained for each co-micronized material prepared are represented.
[0066] Table 2: Percentage of Apixaban Released Relative to the Initial Amount of Apixaban Contained in Tablets
[0067]
[0068] The above results indicated that the dissolution rate of apixaban increased with the decrease of the weight ratio of apixaban / sodium lauryl sulfate.
Embodiment 3
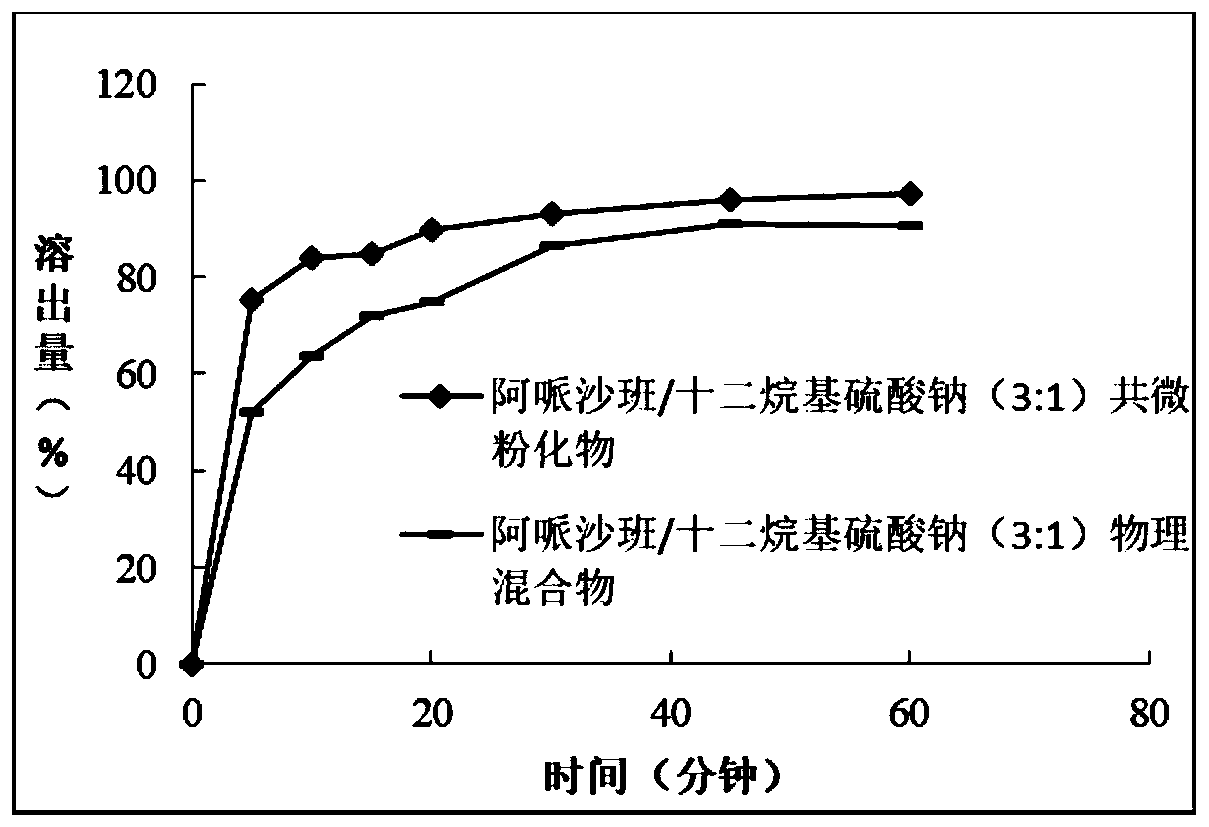
[0069] Embodiment 3: Apixaban / sodium lauryl sulfate co-micronized product mixed with apixaban / sodium lauryl sulfate Comparison of dissolution properties between compounds
[0070] 1. Materials and methods
[0071] Apixaban and sodium lauryl sulfate can be mixed in a mortar and mortar in a weight ratio of 3 / 1 and ground until a homogeneous mixture is obtained.
[0072] The remaining mixture was co-micronized using an airflow milling micronizer, and the obtained micronized product (ie, each tablet containing 2.5 mg of apixaban) was compressed into tablets.
[0073] The dissolution of apixaban from these two types of tablets was studied under the same conditions as described in Example 1 and in the same dissolution medium.
[0074] 2. Results
[0075] The dissolution results obtained for the apixaban / SLS co-micronized and non-micronized apixaban / SLS mixtures (physical mixtures) are presented in Table 3 below and image 3 shown in .
[0076] Table 3: Percentage of Apixaban...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com