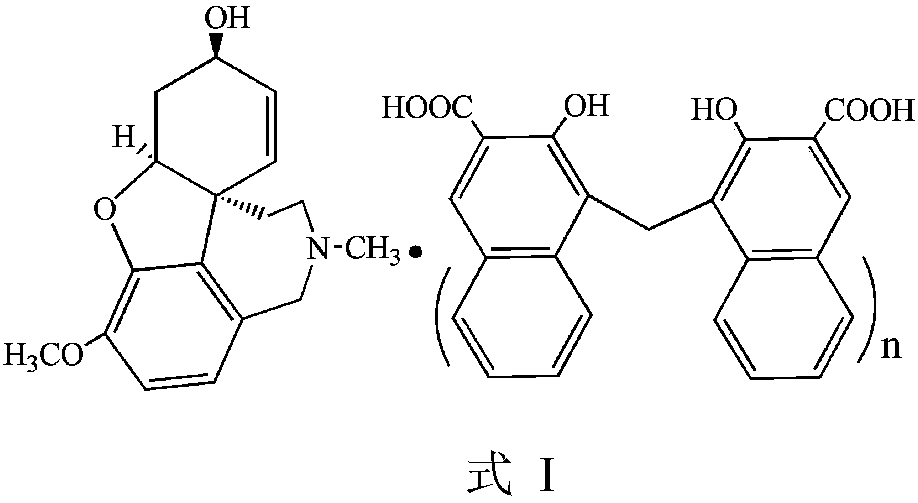
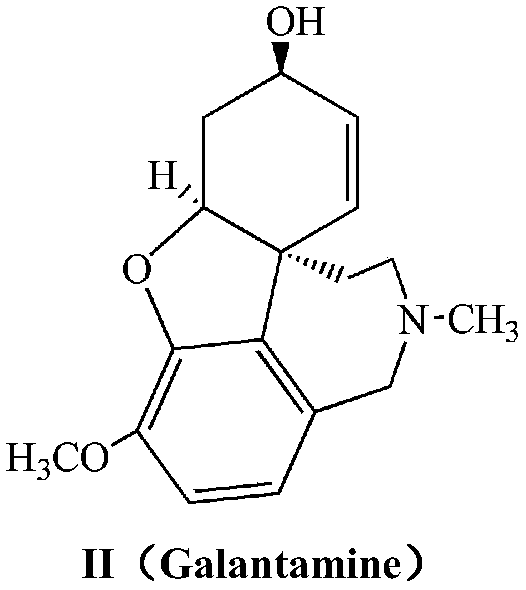
Galanthamine pamoic acid salt and preparation method thereof
A technology of galantamine and pamolate, applied in the field of galantamine pamoate and its preparation, can solve the problems of low solubility property, stable dissolution performance, low solubility property, light resistance, poor stability and the like , to achieve the effects of excellent solubility and stability, high product yield, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
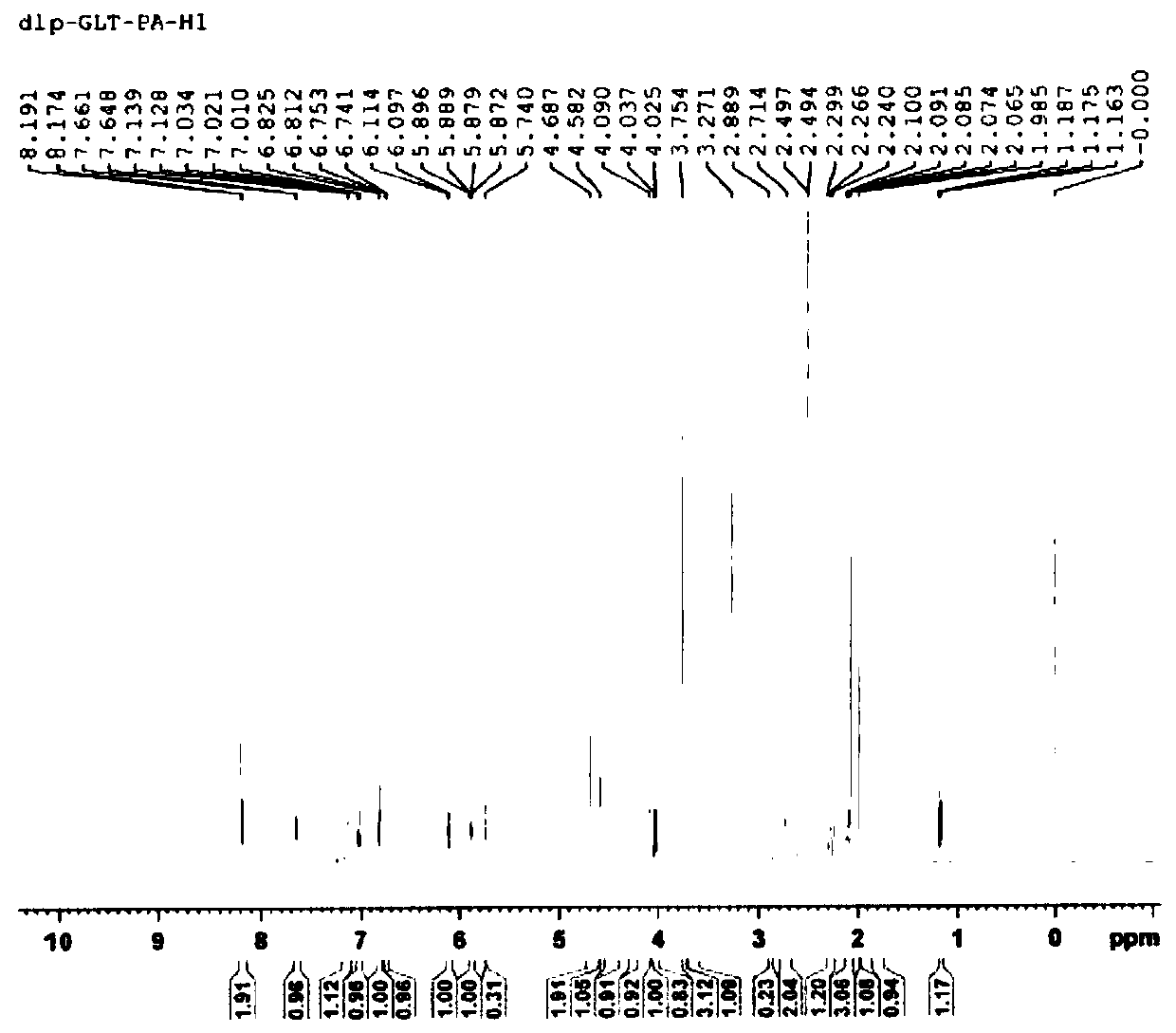
[0033] Dissolve 1.14 g of galantamine hydrobromide in 25 mL of dimethyl sulfoxide, add dropwise 100 mL of a dimethyl sulfoxide solution containing 1.41 g of pamoic acid, and after the drop is complete, stir and react at 25°C for 6 hours; The liquid was cooled to 0-5°C and stood at this temperature for 24 hours, vacuum filtered, and vacuum-dried at 50°C for 12 hours to obtain a light yellow powder with a yield of 78.55% and a purity of 99.91%.
Embodiment 2
[0035] Dissolve 1.14g of galantamine hydrobromide in 25mL of N,N-dimethylformamide, add dropwise 150mL of N,N-dimethylformamide solution containing 2.57g of pamoic acid, dropwise, at 60°C Stir the reaction for 2 hours; after the reaction, cool the reaction solution to 0-5°C and let it stand at this temperature for 48 hours, vacuum filter, and vacuum-dry at 50°C for 12 hours to obtain a light yellow powder with a yield of 75.28% and a purity of is 99.87%.
Embodiment 3
[0037] Dissolve 1.14 g of galantamine hydrobromide in 15 mL of 1,3-dimethyl-2-imidazolinone, and add dropwise 1,3-dimethyl-2-imidazolidinone containing 1.17 g of pamoic acid Solution 150mL, after dripping, stirred at 15°C for 10 hours; put the reaction solution in an environment of 0-5°C for 24 hours, filtered it with suction, and dried it under vacuum at 50°C for 2 hours to obtain a light yellow powder with a yield of 72.35 %, the purity is 99.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com