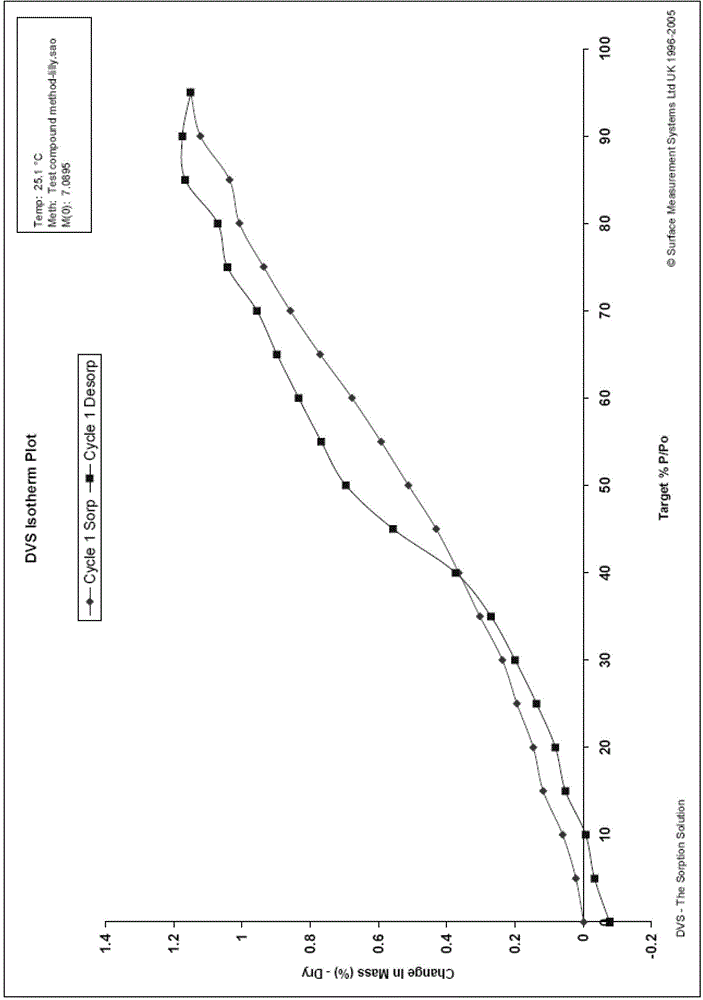
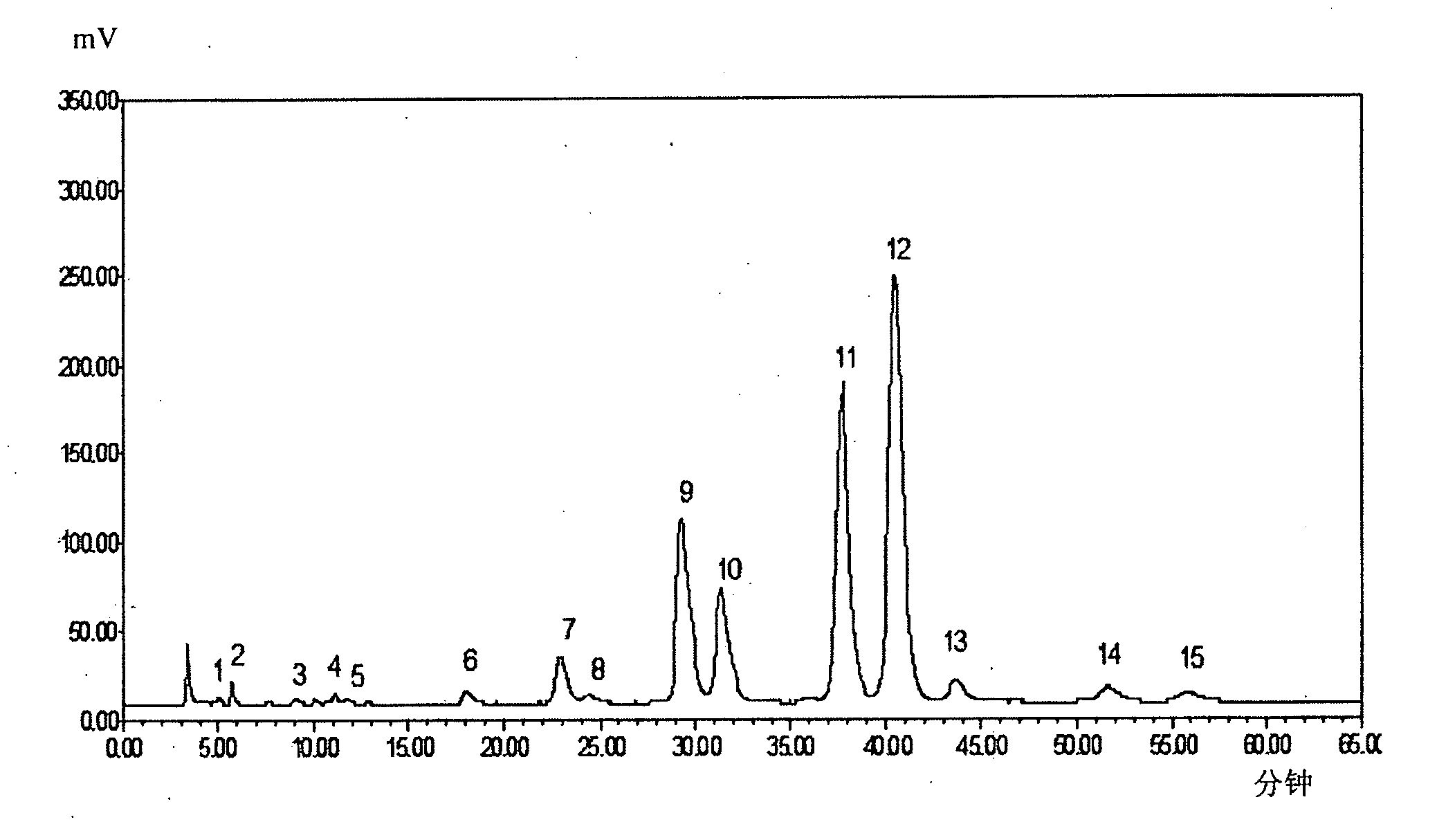
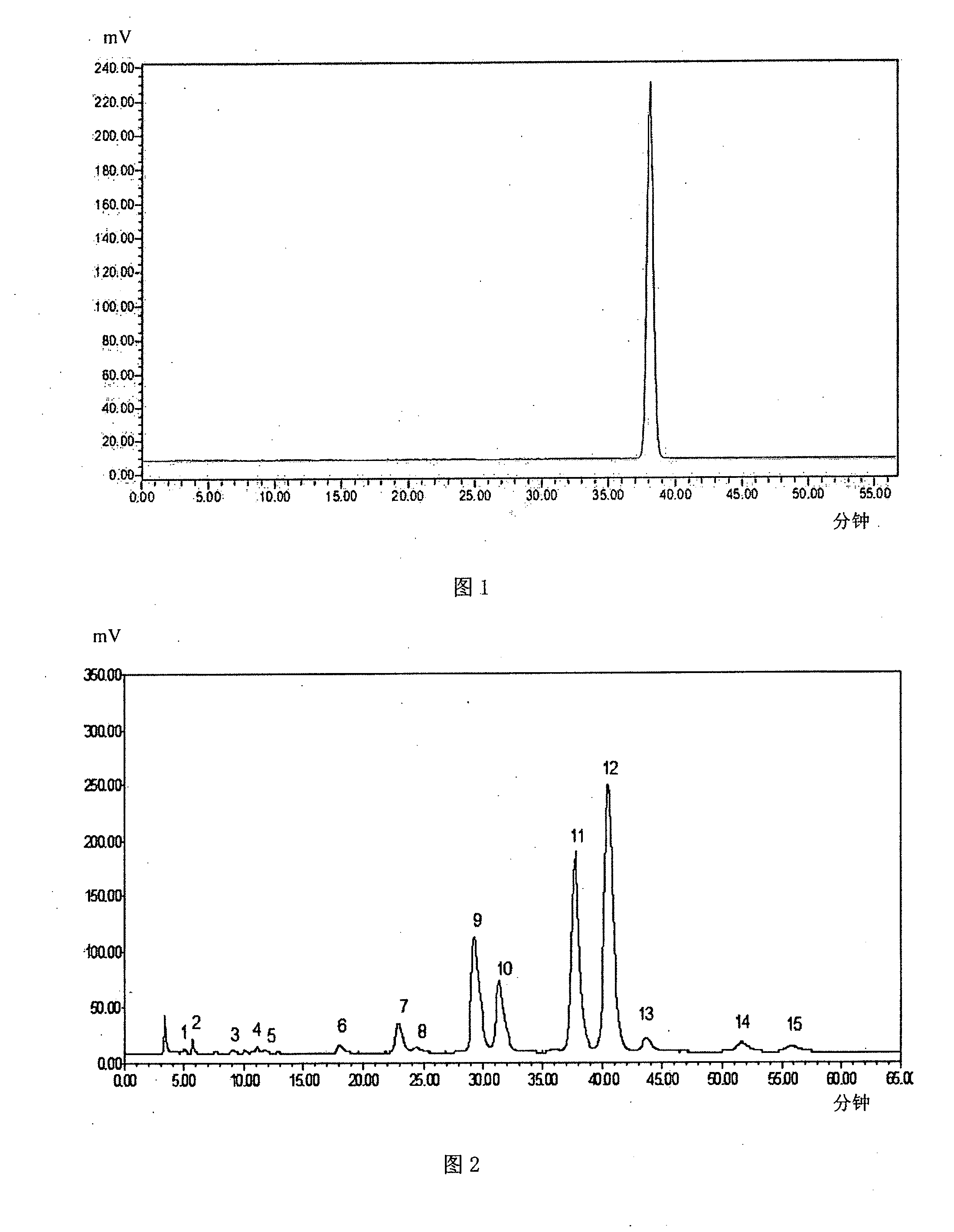
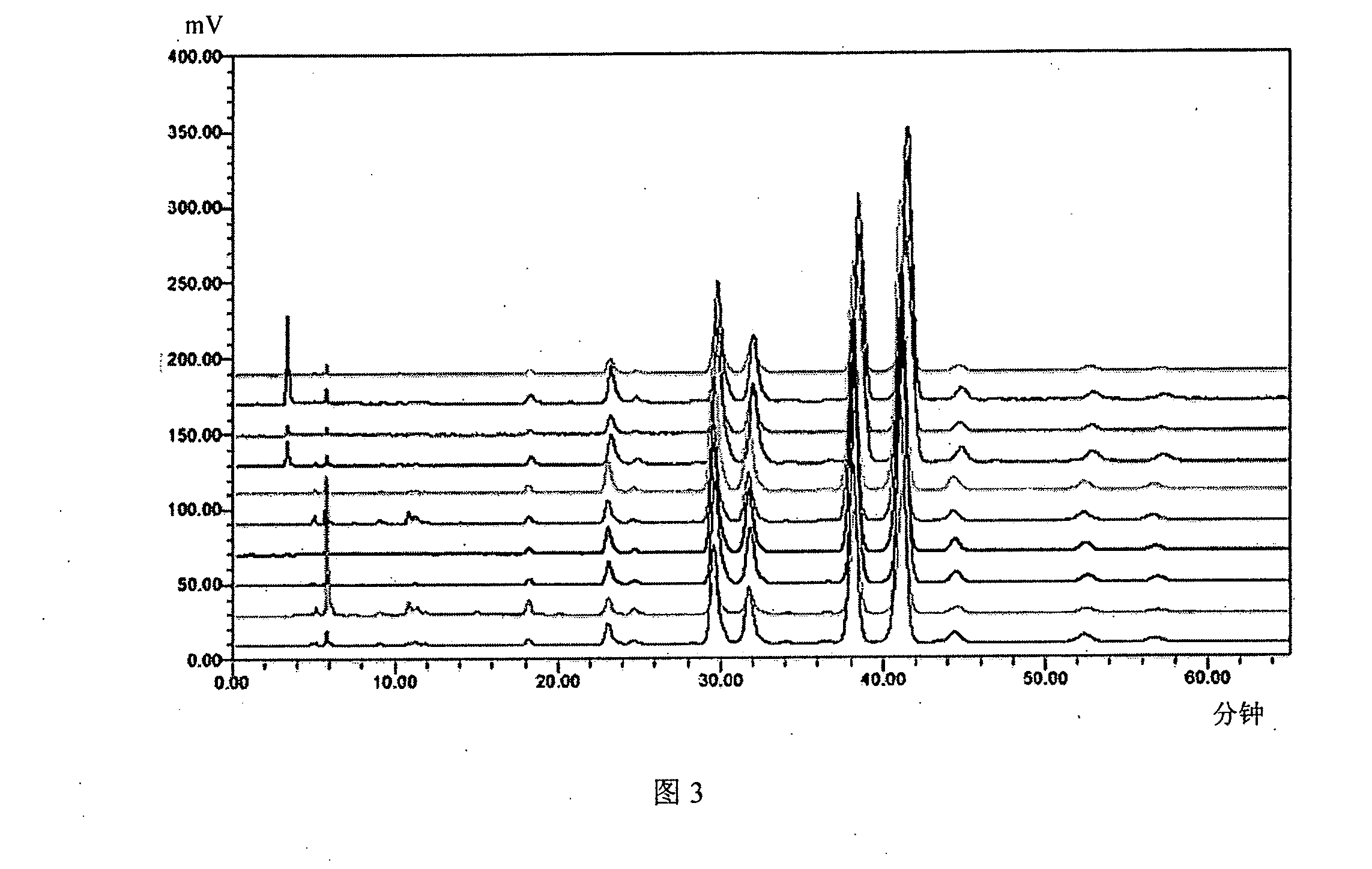
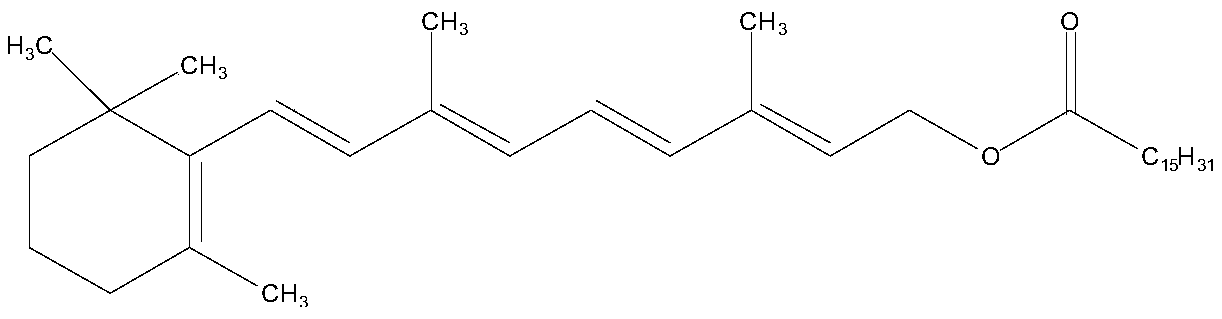
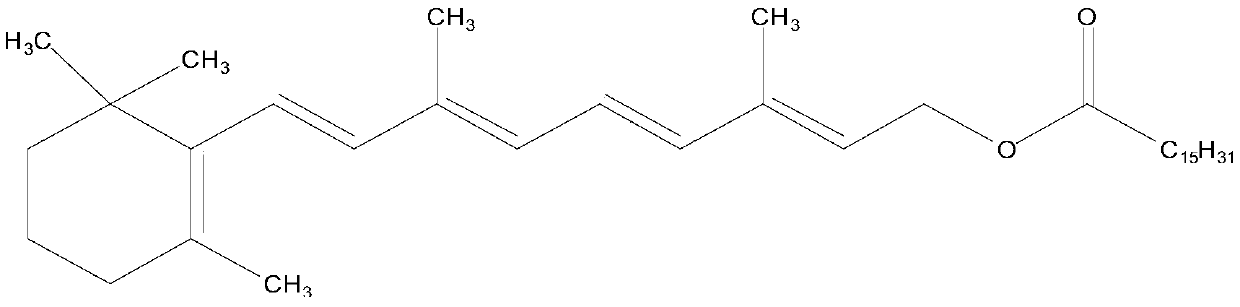
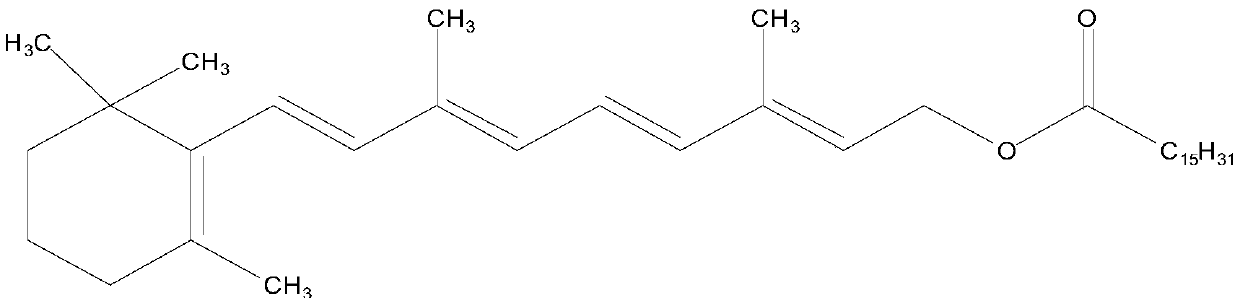
Patents
Literature
32 results about "Palmoxiric Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
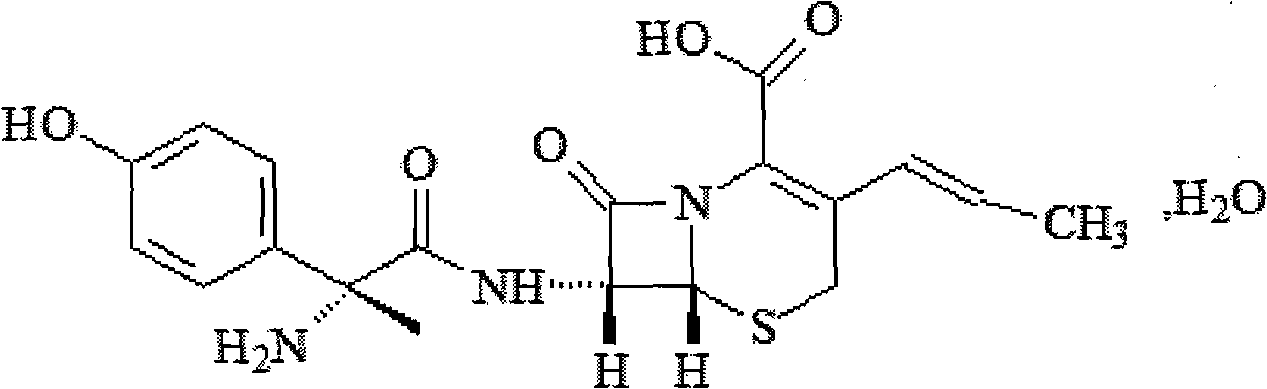
An orally active inhibitor of long-chain fatty acid oxidation with hypoglycemic activity. By inhibiting fatty acid oxidation, palmoxiric acid increases carbohydrate oxidation attributed to the glucose-fatty acid cycle (Randle cycle) resulting in decreased blood glucose levels.
Ruminant animal fat powder and preparation process thereof
InactiveCN102948652AIncrease energy concentrationDoes not affect fermentationAnimal feeding stuffBiotechnologyPhospholipid
The invention belongs to the field of feed processing and particularly provides ruminant animal fat powder. The ruminant animal fat powder is prepared by matching hexadecanoic acid, high-melting-point palm oil, soybean concentrated phospholipids and the like as main fat raw materials with a carrier. The ruminant animal fat powder has no influence on normal physiological activity of microflora in ruminant animal rumen, can pass through the rumen, can be converted into an absorbed form under the actions of chemistry and enzyme in abomasums and duodenum of a digestion system and is digested, absorbed and utilized by small intestine. The product prepared by a formula and a processing technique of the invention is favorable in flowing property and can be mixed to the feed by adopting corresponding dosage stirring according to the requirement of animal nutrition; and the product is convenient and practical and is convenient for storage and transportation.
Owner:SHANDONG ZHONGDA ANIMAL HUSBANDRY GROUP
Surface-treated calcium carbonate and paste-like resin composition containing same
ActiveCN102762500AHigh viscosityImprove thixotropyCalcium/strontium/barium carbonatesPigment treatment with non-polymer organic compoundsStearic acidFatty acid
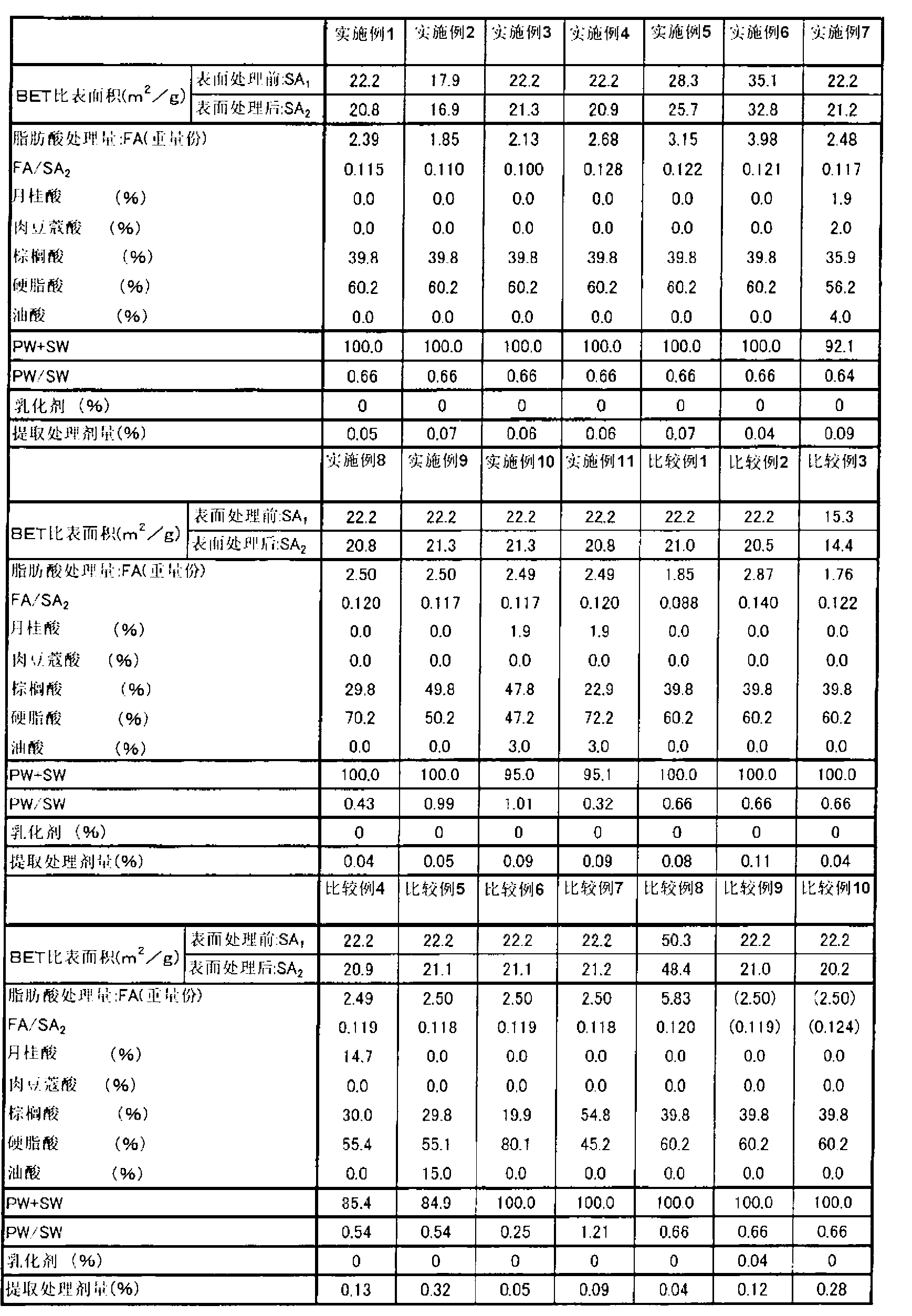
Disclosed are: surface-treated calcium carbonate which can impart a high viscosity and high thixotropic properties to a paste-like resin composition when added to the composition and can impart good mechanical properties and good adhesion properties to a cured product of the paste-like resin composition; and a paste-like resin composition containing the surface-treated calcium carbonate. The surface-treated calcium carbonate is produced by treating the surface of calcium carbonate with a surface-treating agent comprising a sodium salt or a potassium salt of a fatty acid, is characterized in that the sum total of PW and SW (PW + SW) is 90 or more and the ratio of PW to SW (PW / SW) falls within the range from 0.30 to 1.1 inclusive (wherein PW represents the content (wt%) of the sodium salt or the potassium salt of palmitic acid in the surface-treating agent in terms of acid content; and SW represents the content (wt%) of the sodium salt or the potassium salt of stearic acid in the surface-treating agent in terms of acid content), and is also characterized in that SA2 falls within the range from 15 to 48 inclusive and the ratio of FA to SA2 (FA / SA2) falls within the range from 0.095 to 0.135 inclusive (wherein SA2 represents the BET specific surface area (m2 / g) of the surface-treated calcium carbonate; and FA represents the amount of the sodium salt or the potassium salt of the fatty acid to be used for the treatment in terms of acid content relative to 100 parts by weight of calcium carbonate).
Owner:SHIRAISHI KOGYO KAISHA LTD
Medical PVC material adopted for steam sterilization, and preparation method thereof
The invention relates to a medical PVC material adopted for steam sterilization, and a preparation method thereof. The material comprises, by weight, 100 parts of PVC resin, 40-70 parts of a plasticizer DOP, 1.5-2.0 parts of a calcium / zinc heat stabilizer, 6-10 parts of an assistant heat stabilizer and 1.0-1.5 parts of a lubricant. The PVC resin is the polyvinyl chloride resin prepared through a suspension method, and has a polymerization degree of 1000-1300. The calcium / zinc heat stabilizer is a composite, wherein the composite comprises, by weight, 40-60% of calcium stearate, 20-30% of zincpalmitate, 15-30% of hydroxy magnesium / aluminum. The assistant heat stabilizer is epoxidised soyabean oil. The lubricant comprises, by weight, 55-60% of silicone oil and 40-45%of oxidized polyethylene wax. In the prior art, the PVC material is sterilized through the steam sterilization method, the high temperature (121 DEG C) and the long time treatment (30 minutes) during the steam sterilization process can cause degradation and yellowing of the PVC material, the precipitation of the plasticizer can cause clamminess generation of the instrument surface. With the present invention, the problems in the prior art are solved, the heat resistance of the medical PVC material is improved, and the probabilities of the yellowing and the precipitation of the plasticizer after steam sterilization are reduced.
Owner:常州恒方大高分子材料科技有限公司 +2
Prasugrel salt and preparation method thereof
The invention relates to a prasugrel pamoate salt and a preparation method thereof. The prasugrel pamoate salt has a structure represented by the formula II, and has the advantages of good stability, low hygroscopicity, and high safety. Moreover, the preparation method has the advantages of simple operation and good repeatability. The provided prasugrel salt can be used as an anti-platelet drug.
Owner:SHANGHAI SYNCORES TECH INC
The quality control method and application of a kind of ganoderma lucidum spore oil fat emulsion
This invention discloses a kind of quality control method and application of Ganoderma lucidium spore oil fat emulsion. Which includes Ganoderma lucidium spore oil 2˜25%, emulsifier 0.5˜10%, isosmotic agent 0.2˜5%, the remaining content is water and the final pH of Fat emulsion is adjusted to 6˜9. The quality control method of this invention can accurately determine the content of 1,2-oleic-3-palmitic triglyceride and glycerol trioleate in the preparation, and accurately determine the ergosterol content, which serves as the method and basis for quality control of this product. Fingerprints are utilized to grasp the product quality from the overall characteristics of Chromatogram. This invention has clearly defined active ingredients, with excellent bioactivity, and is capable of tumor treatment, while improving the organism's immunity, as well as the life quality of tumor patients undergoing radiotherapy and chemotherapy, and relieving the drug toxicity after treatment. This invention is particularly suitable for both intra-arterial injection and intravenous injection, enabling the Ganoderma lucidium spore oil to directly gain access to the human blood circulation, with short time to onset and complete absorption as well as high security, reliable quality, and low drug toxicity.
Owner:GUANGZHOU HANFANG NATURAL MEDICINE RES & DEV
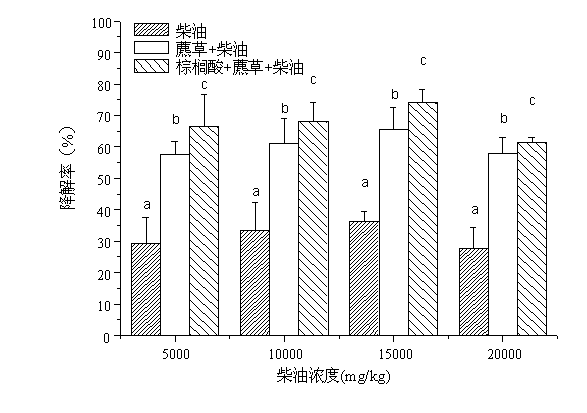
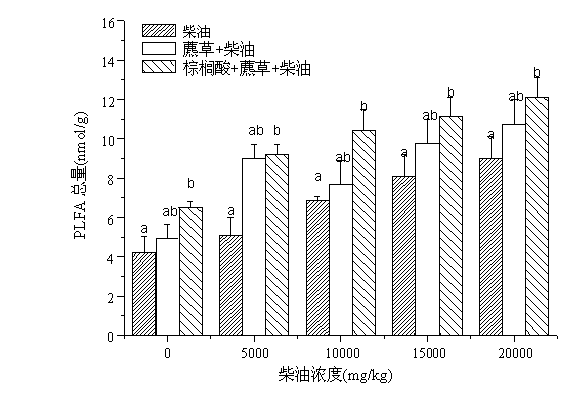
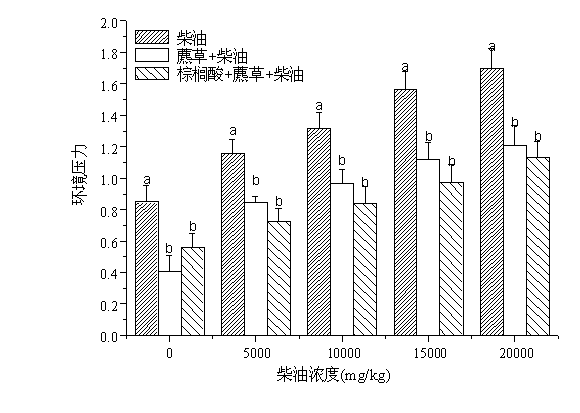
Method for repairing oil polluted wetland soil environment with cetylic acid intensified scirpi
InactiveCN102699017ADoes not affect entity qualityQuality is not affectedClimate change adaptationContaminated soil reclamationPolluted environmentSeedling
The invention relates to a method for repairing an oil polluted wetland soil environment with cetylic acid intensified scirpi. The method practically comprises the application step of transplanting scirpi seedlings to oil polluted wetland soil which is added with cetylic acid, wherein the planting density is 800 to 1000 per m<2>, and the mass ratio of the cetylic acid to the wetland soil is (1000 mg to 5000 mg) to 1 kg. According to the method in which plant intensified with root exudate cetylic acid is creatively used for repairing the oil polluted wetland soil environment, the repairing effect is favorable, the environment is protected, the maneuverability is high, and the cost is low. Therefore, the method has a wide application prospect in the plant repairing field for oil polluted environments.
Owner:SHANGHAI UNIV
Crystal silicon polishing additive and use method thereof for crystal silicon polishing
PendingCN107904663AReduce concentrationImprove polishing effectPolycrystalline material growthAfter-treatment detailsPotassium hydroxideSolar cell
The invention relates to a crystal silicon polishing additive and a use method thereof for crystal silicon polishing and belongs to the technical field of production of solar cells. The crystal silicon polishing additive comprises the following raw material components in percentage by weight: 0.01-3 percent of sodium citrate, 0.01-3 percent of cetylic acid, 0.001-1 percent of hexadecyl trimethylamine and 0.1-2 percent of Tween and the balance of deionized water; when the additive is in use, sodium oxide or potassium hydroxide is required to be added to prepare polishing liquid; and the volumepercent of the additive to an alkaline solution is (1.2:100)-(5:100), the temperature of the polishing liquid is 55-80 DEG C, and the crystal silicon polishing time is 120-300 s. The crystal silicon polishing additive and the use method thereof, provided by the invention, have the beneficial effects that the reaction speed is controlled by utilizing the cetylic acid, the alkali concentration is increased simultaneously, and lattice bases with uniform sizes are obtained; and the sodium citrate has a metal complexing effect, has a cleaning effect on a silicon chip and is low in cost.
Owner:绍兴拓邦新能源股份有限公司
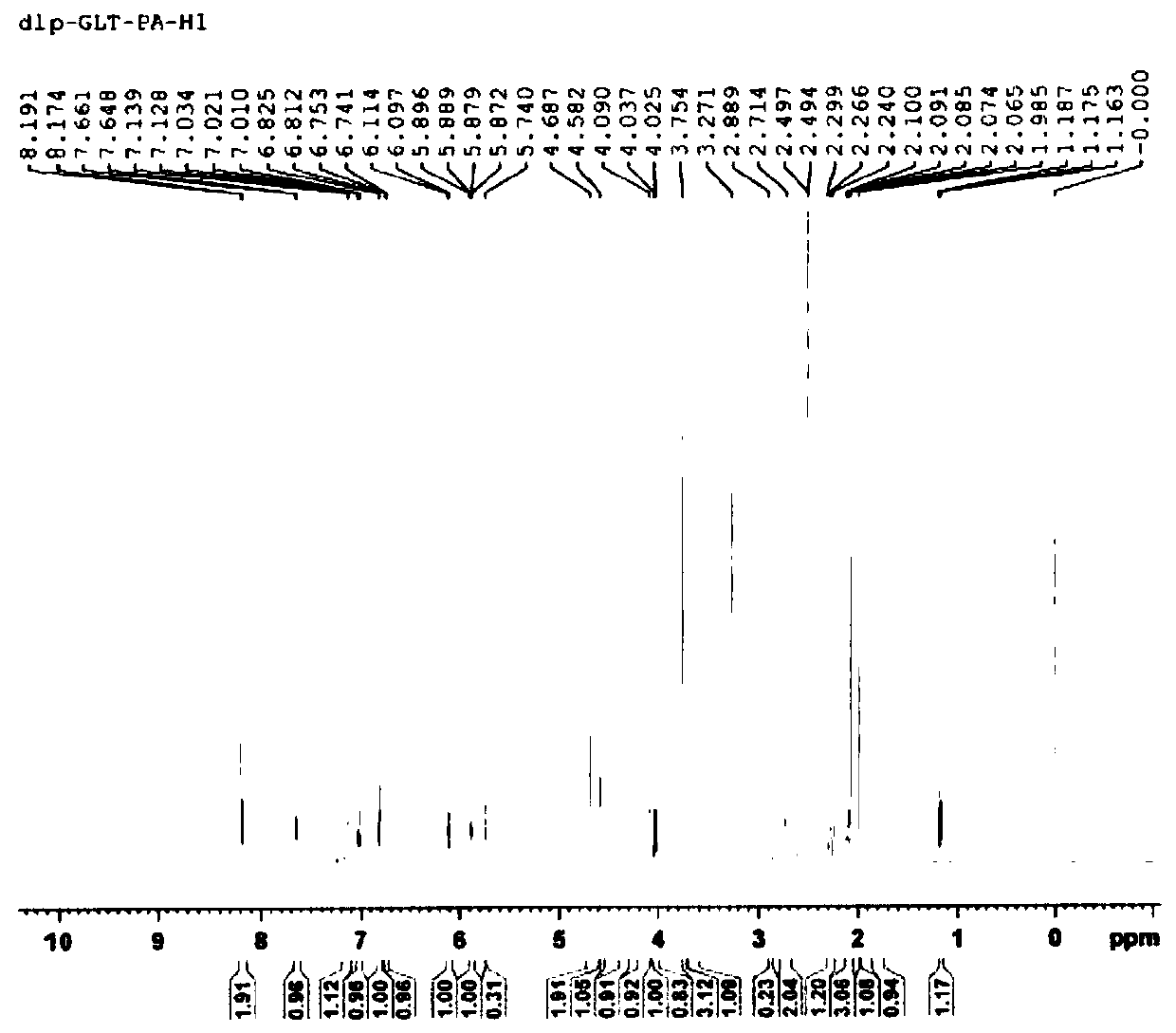
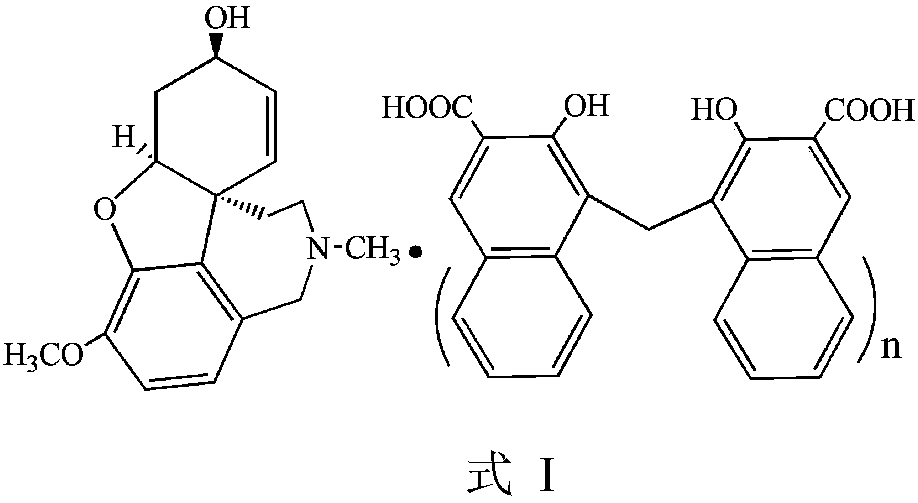
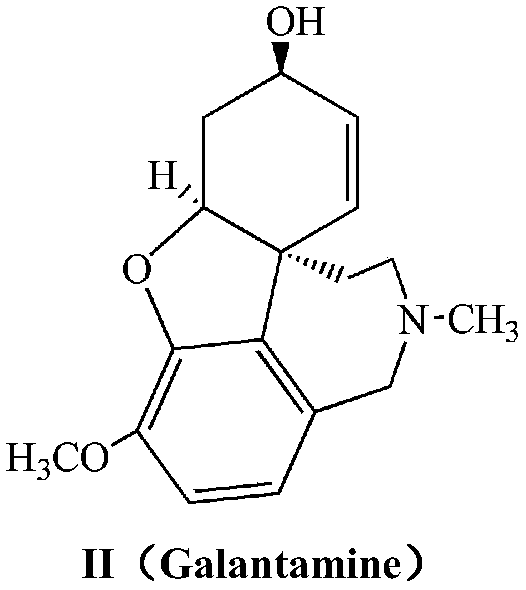
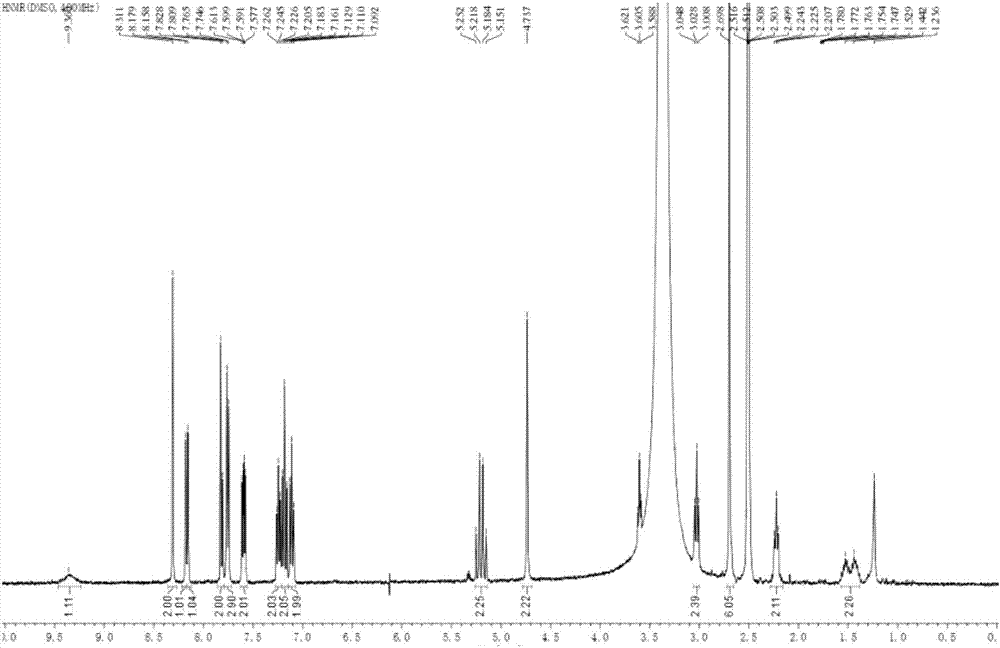
Galanthamine pamoic acid salt and preparation method thereof
ActiveCN111233877AImprove stabilityReduce solubilityNervous disorderOrganic chemistryEngineeringCombinatorial chemistry
The invention provides a galanthamine pamoic acid salt and a preparation method thereof, the compound has a structure shown in formula I, and has good dissolution characteristic and good stability, and a tablet prepared by using the compound as an active ingredient has slow dissolution performance and embodies an excellent slow release effect. In addition, the galanthamine pamoic acid salt provided by the invention has the advantages of simple preparation process, high product yield and high purity, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Citalopram pamoate and crystal form thereof, and preparation method and application thereof
InactiveCN107311968AHigh selectivityLittle side effectsOrganic active ingredientsNervous disorderSolubilityHypodermoclysis
The invention relates to citalopram pamoate and a crystal form thereof, and a preparation method and application thereof, belonging to the technical field of medicine. The citalopram pamoate has a structure as shown in a formula I which is described in the specification. The citalopram pamoate has low solubility, can be used for intramuscular injection or subcutaneous injection when made into a suspension, forms a medicine storehouse in vivo, prolongs in-vivo drug release speed and achieves the purpose of long-acting treatment.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
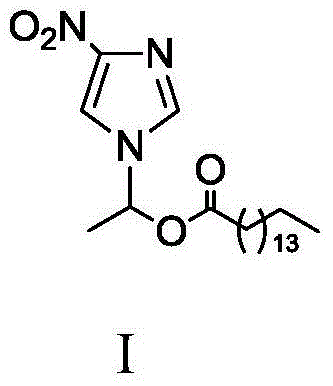
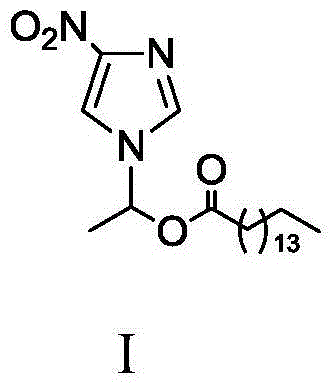
Method for synthesizing 1-(4-nitroimidazolyl)ethyl palmitate on line under catalysis of lipase
ActiveCN104561174AShort reaction timeImprove conversion rateFermentationNitroimidazoleReaction temperature
The invention discloses a method for synthesizing 1-(4-nitroimidazolyl)ethyl palmitate on line under the catalysis of lipase. The method comprises the steps of with 4-nitro-imidazole and vinyl palmitate which are in the molar ratio of 1:(1-8) as raw materials, 0.5-1.0g of lipase Lipozyme TLIM as a catalyst and a DMSO solvent as a reaction solvent, uniformly filling a reaction channel of a microfluidic channel reactor with the lipase Lipozyme TLIM, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; and continuously introducing the raw materials and the reaction solvent to the reaction channel to carry out Markovnikov addition reaction, controlling the reaction temperature at 40-55 DEG C and the reaction time at 20-35 minutes, collecting a reaction solution on line, and carrying out conventional treatment on the reaction solution to obtain 1-(4-nitroimidazolyl)ethyl palmitate. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
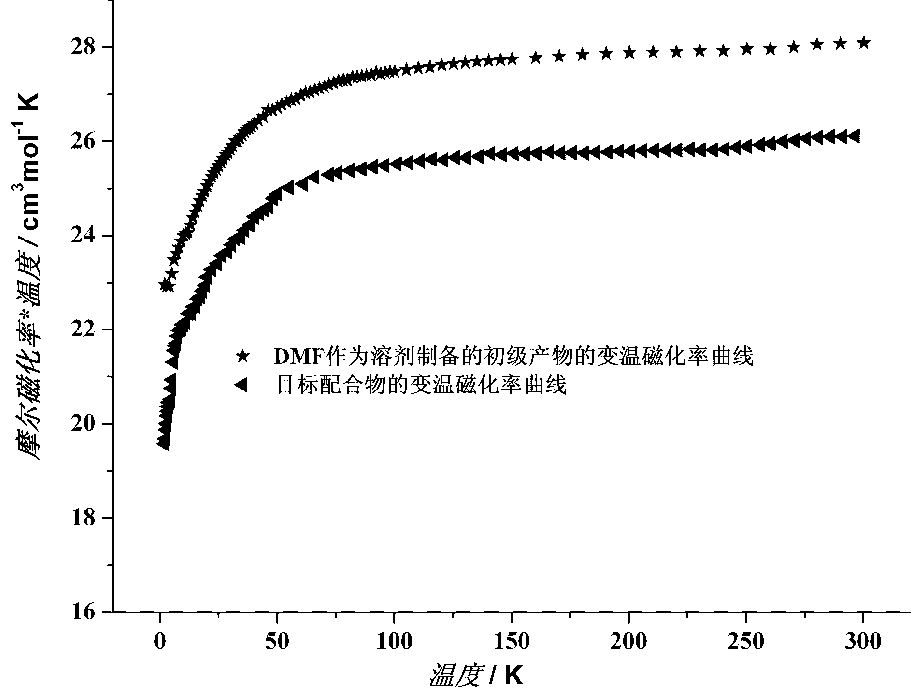
Arrowhead complex material with solvent molecule-responded magnetic and ferroelectric properties and preparation method of arrowhead complex material
InactiveCN106928049AImprove ferroelectric propertiesHigh potential application valueOrganic compound preparationOrganic chemistry methodsSolvent moleculeCoordination complex
Owner:HENAN POLYTECHNIC UNIV
Lurasidone pamoate amorphous substance as well as preparation method and application thereof
ActiveCN111454256AGood physical and chemical stabilityImprove bioavailabilityOrganic active ingredientsNervous disorderLurasidone HydrochlorideBioavailability
The invention relates to the technical field of medicines, and discloses a lurasidone pamoate amorphous substance as well as a preparation method and application thereof. The method comprises the following steps: (1) dissolving lurasidone hydrochloride and pamoic acid disodium salt, or lurasidone and pamoic acid in tetrahydrofuran to obtain a first mixed solution; (2) stirring for 2 to 3 h at 55 to 60 DEG C for reaction to obtain a second mixed solution; and (3) carrying out spin-drying on the second mixed solution at 55-60 DEG C, and carrying out precipitation by taking water as a precipitation solvent. The pamoic acid lurasidone amorphous substance prepared by the preparation method is different from lurasidone hydrochloride in the prior art in form; the equilibrium solubility of the first amorphous substance and the second amorphous substance of the pamoic acid lurasidone prepared by the method is 50.69-635 [mu]g / mL and 29-361 [mu]g / mL respectively between pH 6-7.8, so that the solubility of lurasidone hydrochloride can be remarkably improved, and the oral bioavailability of lurasidone is improved.
Owner:HUBEI UNIV OF CHINESE MEDICINE
Solid cefprozi lipid nanoparticle preparation
InactiveCN102327226AImprove solubilityGood controlled release effectAntibacterial agentsPowder deliverySide effectGlycerol
The invention discloses a solid cefprozi lipid nanoparticle preparation and a preparation method thereof. Cefprozi, palmitic acid and glycerol tristearate are dissolved in an organic solvent to form an oil phase; and with a water solution of polyoxyethylene 40 stearate as water phase, the cefprozi is entrapped in a solid lipid nanoparticle to obtain the solid cefprozi lipid nanoparticle preparation by adopting a mixed emulsifying and high-pressure uniformly-emulsifying combined method. The solid lipid nanoparticle preparation provided by the invention has the advantages of high drug loading amount, uniformity in grain size, long reservation time of a medicament in blood circulation and better slowly-releasing and controlled-release effects; and the quality of a preparation product is improved and toxic or side effects are reduced. In addition, equipment used by a preparation method is simple, easy for operation and suitable for industrial large-scale production.
Owner:HAINAN MEIDA PHARMA
Bacteriostatic benzoic acid type enteric sustained-release acidifier and preparation method thereof
InactiveCN109043145AAlleviate pungent odorAcidification range extensionAccessory food factorsWorking-up animal fodderBenzoic acidAcrylic resin
The invention relates to a bacteriostatic benzoic acid type enteric sustained-release acidifier and a preparation method thereof. The bacteriostatic benzoic acid type enteric sustained-release acidifier is prepared from the following materials by weight percent: 10-60% of benzoic acid, 5-10% of lactic acid, 5-15% of fumaric acid, 5-10% of silica, 5-10% of microcrystalline cellulose and / or corn starch, 5-10% of beta-cyclodextrin and / or maltodextrin, and 10-40% of stearic acid and / or palmitic acid or acrylic resin. The preparation method comprises the following steps: S1: carrying out pretreatment on benzoic acid; S2: carrying out solid-liquid mixing; S3: carrying out solid-solid mixing; S4: tabletting and granulating; S5: carrying out three-stage screening; S6: preparing a coating material;S7: coating; S8: atomizing the coating material, and spraying into a coating machine for coating; after the coating is completed, discharging the material into an automatic screening machine, and screening to obtain the finished product. The product prepared by the method can be released in sections in the gastrointestinal tract, so that the benzoic acid is prevented from being completely absorbed by the anterior segment of the digestive tract, and the acidification range is effectively expanded; furthermore, the preparation process is simple to operate, the degree of automation is high, andthe production cost can be greatly reduced.
Owner:上海酸能埃赛生物科技有限公司
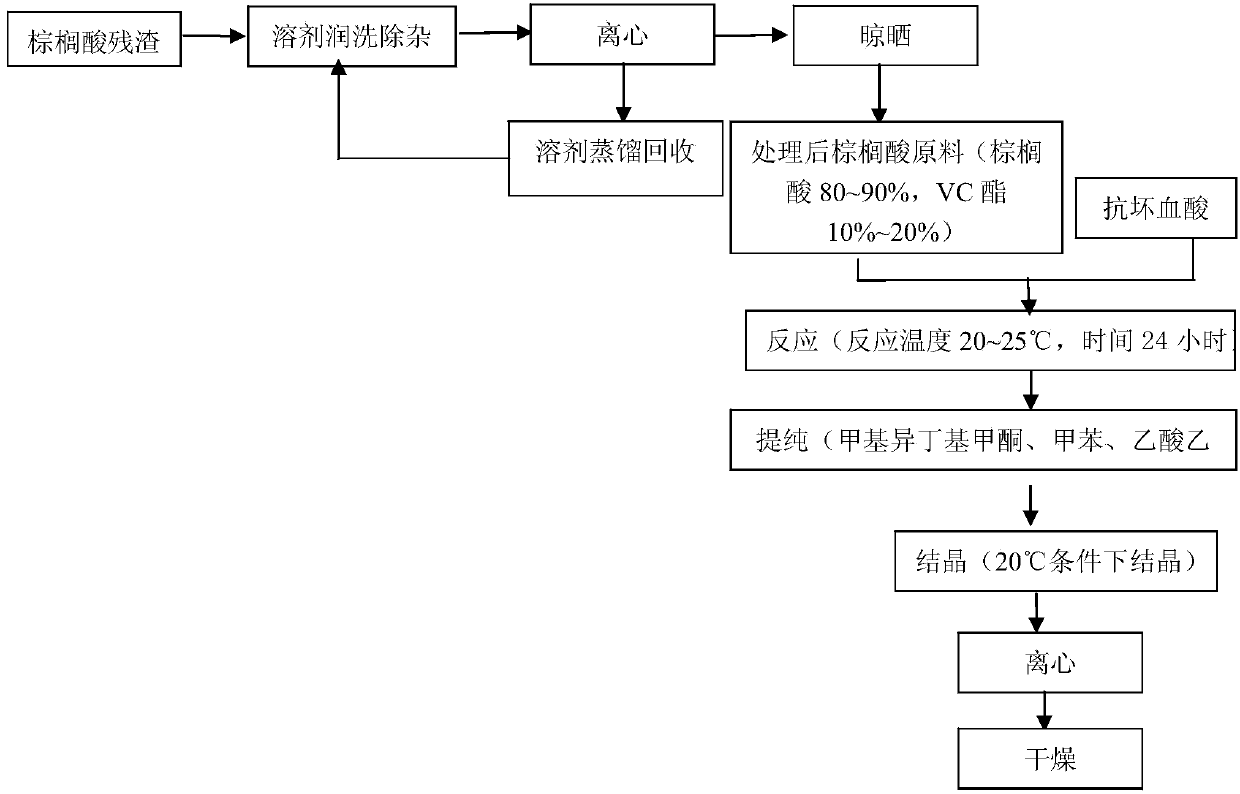
Treatment method of palmitic acid residues
The invention relates to the technical field of industry engineering, and particularly relates to a treatment method of palmitic acid residues generated in the technological process of ascorbyl palmitate production. The treatment method comprises the following steps: firstly using a solvent for moistening and washing the palmitic acid residues so as remove impurities in the palmitic acid residues, then sequentially carrying out working procedures such as centrifuging, airing, putting ascorbic acids for reaction, purifying, crystallizing and drying, and finally converting the palmitic acid residues to the ascorbyl palmitate, wherein solvent filtrate obtained in the centrifuging working procedure returns to the moistening and washing impurity removal working procedure. The treatment method has the advantages that waste residues generated in a traditional preparation process of the ascorbyl palmitate are treated and prevented from polluting environments on the one hand, useful materials in the waste residues are utilized on the other hand, so that waste is turned into wealth, the yield of products in the ascorbyl palmitate production is increased by above 10%, and the cost is reduced by 10%-20%.
Owner:GUANGDONG GUANGYI TECH IND
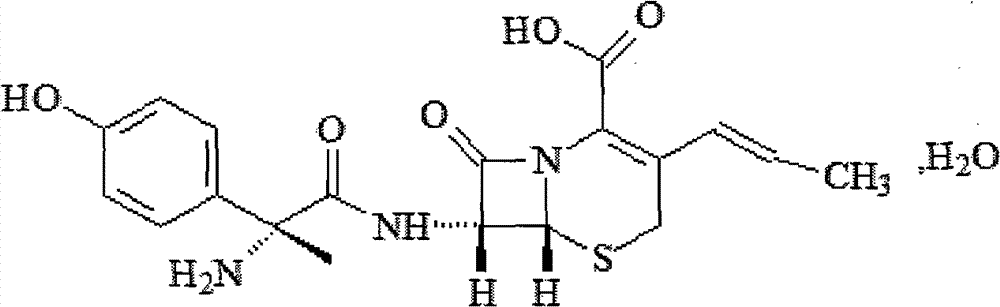
Powder injection of the donepezil semi palmoxiric acid salt, composition containing same and preparation method therefor
ActiveUS11197850B2Reduce stimulationImprove liquidityNervous disorderOrganic chemistryEngineeringPalmitic acid
A powder injection of a donepezil semi palmoxiric acid salt, a composition containing the same and a preparation method therefor. The powder injection contains donepezil semi palmoxiric acid salt crystals, and the average grain size of the crystals ranges from 0.5 μm to 100 μm.
Owner:SHANGHAI SYNERGY PHARMA SCI CO LTD +1
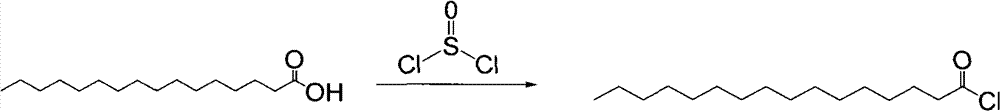
Method for preparing palmitoyl chloride
ActiveCN101863753BAvoid heavy useRaw materials are easy to getOrganic compound preparationCarboxylic compound preparationPalmitoyl chlorideProcess equipment
The invention relates to the technical field of preparation of palmitoyl chloride, in particular to a method for preparing the palmitoyl chloride from palmitic acid and thionyl chloride. In the method, the palmitic acid and the thionyl chloride are reacted at the temperature of between 60 and 75 DEG C for 0.5 to 2 hours in a magnetic stirring reaction kettle in the presence of organic amine to form the palmitoyl chloride. The method has the advantages of readily available raw materials, simple process equipment, low energy consumption, low cost, particularly quick reaction in the whole process, and easy industrial production.
Owner:GUANGDONG GUANGYI TECH IND
Ruminant animal fat powder and preparation process thereof
InactiveCN102948652BIncrease energy concentrationDoes not affect fermentationAnimal feeding stuffBiotechnologyPhospholipid
The invention belongs to the field of feed processing and particularly provides ruminant animal fat powder. The ruminant animal fat powder is prepared by matching hexadecanoic acid, high-melting-point palm oil, soybean concentrated phospholipids and the like as main fat raw materials with a carrier. The ruminant animal fat powder has no influence on normal physiological activity of microflora in ruminant animal rumen, can pass through the rumen, can be converted into an absorbed form under the actions of chemistry and enzyme in abomasums and duodenum of a digestion system and is digested, absorbed and utilized by small intestine. The product prepared by a formula and a processing technique of the invention is favorable in flowing property and can be mixed to the feed by adopting corresponding dosage stirring according to the requirement of animal nutrition; and the product is convenient and practical and is convenient for storage and transportation.
Owner:SHANDONG ZHONGDA ANIMAL HUSBANDRY GROUP
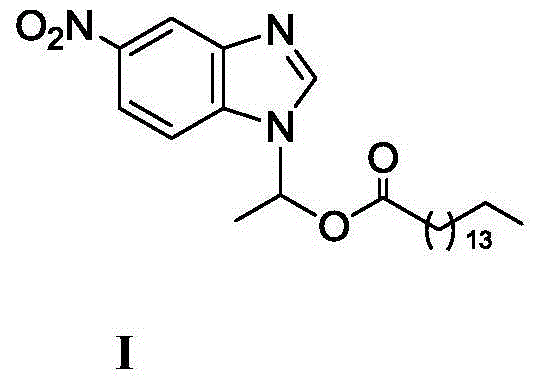
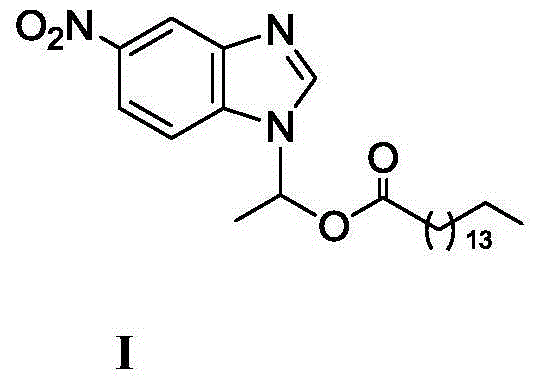
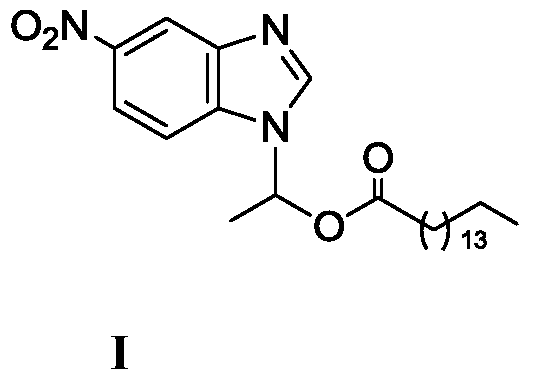
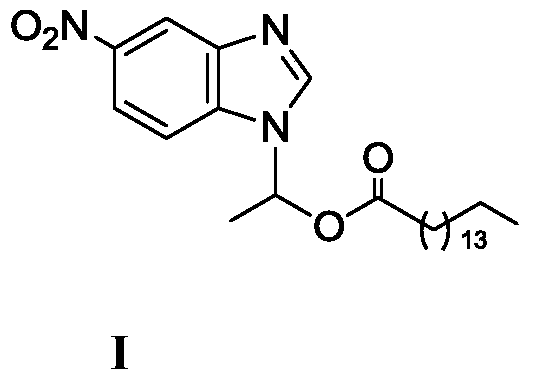
Method for synthesizing 1-(6-nitrobenzimidazolyl)ethyl palmitate on line under catalysis of lipase
ActiveCN104561173AShort reaction timeImprove conversion rateFermentationEthyl palmitateReaction temperature
The invention discloses a method for synthesizing 1-(6-nitrobenzimidazolyl)ethyl palmitate on line under the catalysis of lipase. The method comprises the steps of with 6-nitro-benzimidazole and vinyl palmitate which are in the molar ratio of 1:(1-8) as raw materials, 0.5-1.0g of lipase Lipozyme TLIM as a catalyst and a DMSO solvent as a reaction solvent, uniformly filling a reaction channel of a microfluidic channel reactor with the lipase Lipozyme TLIM, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; and continuously introducing the raw materials and the reaction solvent to the reaction channel to carry out Markovnikov addition reaction, controlling the reaction temperature at 40-55 DEG C and the reaction time at 20-35 minutes, collecting a reaction solution on line, and carrying out conventional treatment on the reaction solution to obtain 1-(6-nitrobenzimidazolyl)ethyl palmitate. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Aluminum-based composite heat dissipation material containing glass wool and used for light LED (light-emitting diode)
The invention relates to a lamp heat dissipation material, in particular to an aluminum-based composite heat dissipation material containing glass wool and used for a light LED (light-emitting diode). The heat dissipation material is prepared from the following raw materials in parts by weight: 80-83 parts of aluminum, 4-5 parts of copper, 2-3 parts of iron, 6-8 parts of aluminum nitride, 9-10 parts of titanium-silicon powder, 2-4 parts of glass wool, 3-5 parts of zinc oxide, 1-3 parts of chrome iron ores, 1-2 parts of manganese oxide ores, 0.2-0.3 part of hexadecyl trimethyl ammonium bromide, 6-8 parts of aluminum hydroxide, 1-2 parts of palmitic acid and 4-5 parts of auxiliaries. The heat dissipation material integrates advantages of raw materials such as the aluminum, the copper, the iron, the aluminum nitride, the glass wool, and the like, and has good heat conduction and insulating property; after being subjected to soaking treatment in a palmitic acid solution, the titanium silicon powder and the glass wool are more liable to be compatible with other raw materials so as to achieve good heat-absorbing and insulating properties. The heat dissipation material prepared in the invention is easy to process and form; and a prepared finished product is smooth and compact in surface, low in weight, high in strength and high in heat conduction coefficient, so that the service lives of lamps are effectively prolonged.
Owner:RUIHUA HEFEI ELECTRONICS TECH
A kind of liposome drug carrier and its preparation method and application
ActiveCN106361701BIncreased ability to enter cellsHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolFreeze-drying
The invention belongs to the technical field of drug preparation development, in particular to a liposome drug carrier and a preparation method and application thereof. The liposome drug carrier includes the following ingredients: cetylic acid and cholesterol. By freeze drying or rotary drying the ingredients transform into membranes. Through the subsequent hydration and filming the final products are acquired. The liposome drug carrier can automatically form lipid microspheres which are quite stable. At the same time the drug carrier preserves the advantages of traditional liposome, namely, the drug carrier has a structure similarity to the bimolecular lamellar membrane structure of biological plasma membrane. The drug carrier has the advantages of excellent biological adaptability, stability, simple production and high encapsulation efficiency and the like. The liposome drug carrier can be used to carry biphosphonate drugs with a consistent and unified particle diameter, high encapsulation efficiency and high stability. The carrier has negative charges and can further enhance the ability that the drug enters cells. Compared with traditional lecithin liposome the carrier enhances the effects on macrophages.
Owner:SHANGHAI JIAO TONG UNIV
Solid cefprozi lipid nanoparticle preparation
InactiveCN102327226BImprove solubilityGood controlled release effectAntibacterial agentsOrganic active ingredientsSide effectGlycerol
The invention discloses a solid cefprozi lipid nanoparticle preparation and a preparation method thereof. Cefprozi, palmitic acid and glycerol tristearate are dissolved in an organic solvent to form an oil phase; and with a water solution of polyoxyethylene 40 stearate as water phase, the cefprozi is entrapped in a solid lipid nanoparticle to obtain the solid cefprozi lipid nanoparticle preparation by adopting a mixed emulsifying and high-pressure uniformly-emulsifying combined method. The solid lipid nanoparticle preparation provided by the invention has the advantages of high drug loading amount, uniformity in grain size, long reservation time of a medicament in blood circulation and better slowly-releasing and controlled-release effects; and the quality of a preparation product is improved and toxic or side effects are reduced. In addition, equipment used by a preparation method is simple, easy for operation and suitable for industrial large-scale production.
Owner:HAINAN MEIDA PHARMA
Novel sunitinib salts and preparing method thereof
The invention relates to novel medicinal sunitinib salts and a preparing method thereof. Sunitinib reacts with adipic acid, succinic acid, and pamoic acid in different solvent systems to respectively produce sunitinib adipate, sunitinib hemisuccinate, and sunitinib hemipamoate. The salts are both stable in the crystal form.
Owner:SHANGHAI SYNCORES TECH INC
A kind of paclitaxel palmitate liposome and preparation method thereof
ActiveCN105853403BAvoid allergic reactionsImprove anti-tumor effectOrganic active ingredientsPowder deliveryPolyoxyethylene castor oilLipolysis
The invention belongs to the technical field of medicine, and specifically relates to a paclitaxel palmitate liposome and a preparation method thereof, wherein paclitaxel palmitate is obtained by esterifying palmitic acid and the 2' hydroxyl of paclitaxel, and belongs to the pro-paclitaxel liposome medicine. The prodrug fundamentally changes the problem of poor fat solubility of paclitaxel, and substantially solves the problem of poor druggability of paclitaxel nano-preparations. Aiming at the special physical and chemical properties of paclitaxel palmitate, the present invention has carried out matching research on the composition of the prescription and the preparation process, and developed a polyoxyethylene castor oil-free, safe, stable quality and simple preparation process in the true sense. Paclitaxel palmitate liposomes have laid a solid foundation for the further research and application of paclitaxel in the field of anti-tumor.
Owner:SHANGHAI WEI ER BIOPHARM TECH CO LTD +3
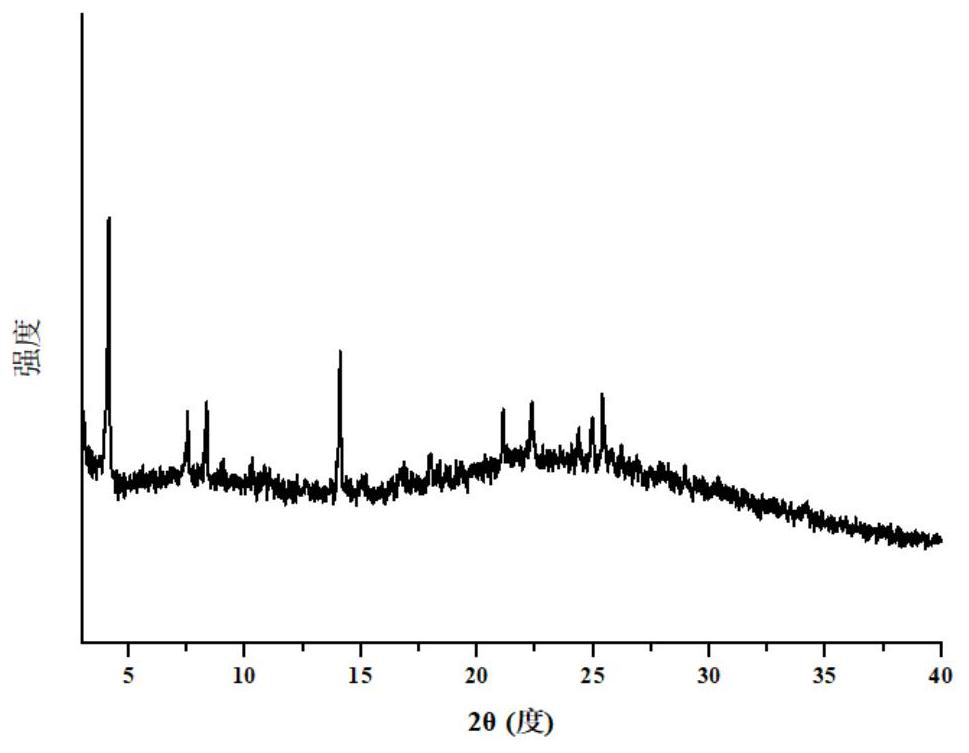
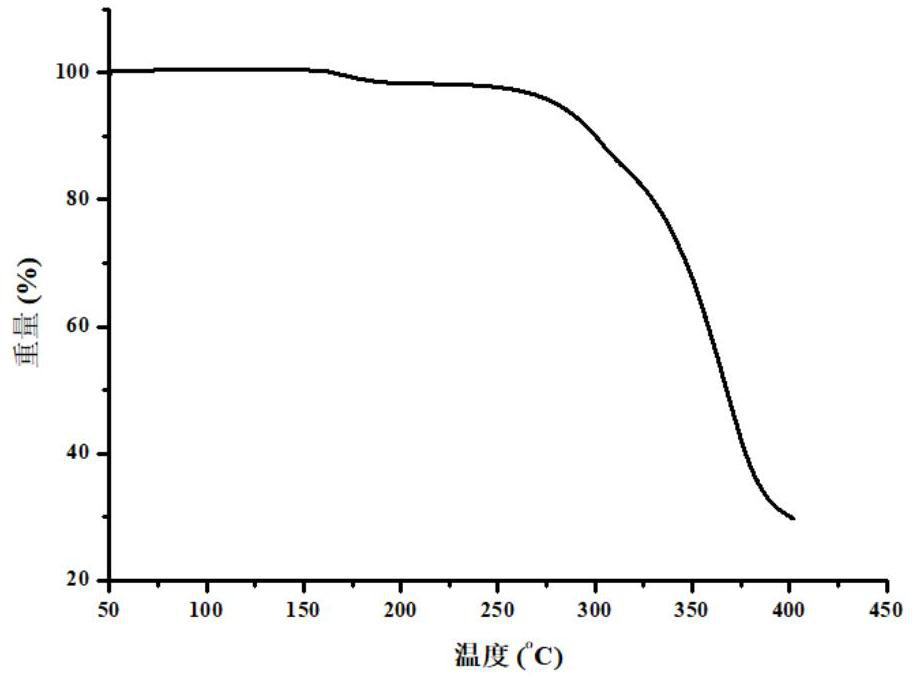
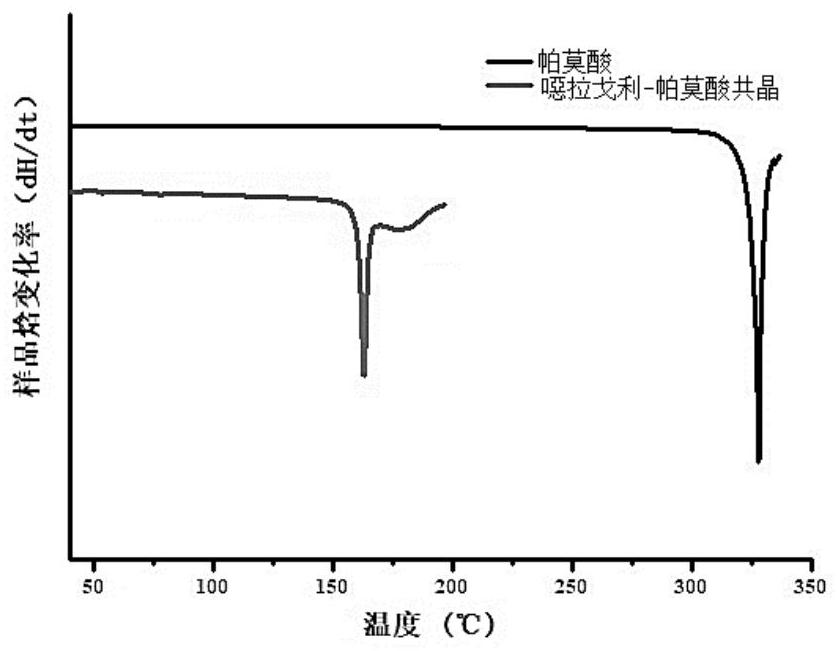
Pharmaceutical co-crystal of elagolix and palmoxiric acid and preparation method of pharmaceutical co-crystal
PendingCN112194635AImprove stabilityHigh crystal purityOrganic active ingredientsAntipyreticHigh humidityPharmaceutical drug
The invention discloses a pharmaceutical co-crystal of elagolix and palmoxiric acid and a preparation method of the pharmaceutical co-crystal, wherein the crystal form has high stability under the conditions of high temperature, high humidity and illumination, and compared with a raw material medicine, the crystal form has higher purity.
Owner:国家卫生健康委科学技术研究所
Enzyme-catalyzed method for preparing vitamin A palmitate
The invention provides a method for preparing vitamin A palmitate by enzyme catalysis, and relates to a synthesis method of vitamin A palmitate. The method comprises the following steps of (1) dissolving the vitamin A palmitate and low-grade fatty alcohol palmitate into an organic solvent, and adding lipase; (2) performing vacuum pumping from a condenser, performing enzyme catalysis ester exchange reaction, and separating out a reaction byproduct of low-grade fatty alcohol acetate and an organic solvent through a rectifying column; (3) removing lipase from reaction material liquid, adding an adsorbent for adsorbing remained low-grade fatty alcohol palmitate, and filtering out the vitamin A palmitate solution; and (4) performing organic solvent reduced pressure evaporation to obtain the vitamin A palmitate. The oxidation cannot easily occur; meanwhile, the enzyme catalysis ester exchange is used during the reaction; the reaction conditions are mild; the side reactions can be effectively inhibited; the operation is simple; the reaction yield is high; and the product quality is high.
Owner:XIAMEN KINGDOMWAY VI TAMIN INC +1
Method for preparing hexadecanoic acid direactive glyceride
InactiveCN101696165BHigh in monoglyceridesIncrease contentOrganic compound preparationCarboxylic acid esters preparationCooking & bakingGLYCERYL PALMITATE
The invention provides a method for preparing hexadecanoic acid direactive glyceride, which comprises the following steps of: taking a certain amount of TiCl14 solution; fully stirring and hydrolyzing the TiCl14 solution by 12 percent dilute ammonia water until the solution becomes alkalescent; finishing deposition; standing the solution for 24h, and then filtering the solution; washing deposits by de-ionized water until no chloride ions exist; drying the washed deposits in a baking oven; grinding the dried TiO2 into fine powder; soaking the fine powder in concentrated sulfuric acid and filtering the solution; drying the soaked and filtered fine powder in the baking oven by heating; extracting the dried fine powder and activating the fine powder in a muffle furnace at a certain temperature to obtain solid super acid; undergoing an esterification reaction at a certain temperature by using the hexadecanoic acid and glycerin as raw materials and the solid super acid as a catalyst to obtain mixed ester of the hexadecanoic acid glyceride; thermally filtering the mixed ester after the reaction ends to remove the catalyst; and using ethyl alcohol as a solvent for extracting for two timesso as to obtain the hexadecanoic acid direactive glyceride. The hexadecanoic acid direactive glyceride prepared by the method has high content and strong emulsifying capacity.
Owner:南通欧通置业有限公司
A kind of lipase-catalyzed method for synthesizing 1-(6-nitrobenzimidazolyl) ethyl palmitate on-line
ActiveCN104561173BShort reaction timeImprove conversion rateFermentationReaction temperatureEthyl palmitate
The invention discloses a method for synthesizing 1-(6-nitrobenzimidazolyl)ethyl palmitate on line under the catalysis of lipase. The method comprises the steps of with 6-nitro-benzimidazole and vinyl palmitate which are in the molar ratio of 1:(1-8) as raw materials, 0.5-1.0g of lipase Lipozyme TLIM as a catalyst and a DMSO solvent as a reaction solvent, uniformly filling a reaction channel of a microfluidic channel reactor with the lipase Lipozyme TLIM, wherein the internal diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the reaction channel is 0.5-1.0m long; and continuously introducing the raw materials and the reaction solvent to the reaction channel to carry out Markovnikov addition reaction, controlling the reaction temperature at 40-55 DEG C and the reaction time at 20-35 minutes, collecting a reaction solution on line, and carrying out conventional treatment on the reaction solution to obtain 1-(6-nitrobenzimidazolyl)ethyl palmitate. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Method for treating sulfur wastewater
InactiveCN106186494APlay a role in decolorizationReduce sulfur contentEnergy based wastewater treatmentMultistage water/sewage treatmentPolystyreneStearate
The invention discloses a method for treating sulfur wastewater. The method includes the steps that formyl polystyrene resin and methyl palmitate are added into the sulfur wastewater, the mixture is reacted at the temperature of 90 DEG C to 120 DEG C for 1 h to 2 h, and a reaction solution A is obtained; the PH value of the reaction solution is adjusted to be 8.0 to 9.0, and the reaction solution is subjected to negative-pressure concentration through a triple effect evaporator to allow the density of the reaction solution A to be 1.5 to 2.0 times that of water; then the obtained reaction solution is cooled in a crystallization separator to be at the temperature of 10 DEG C to 15 DEG C, crystals and liquid are separated, and mixed liquor B is obtained; active carbon loading polyacrylamide, ferric stearate and aluminum acrylate is added into the mixed liquor B, and the mixture is stirred and reacted for 2 h to 3 h at the temperature of 130 DEG C to 150 DEG C at the rotating speed of 500 r / min to 650 r / min; then molecular sieves loading diethyl carbonate are added, the mixture is stirred for 30 min to 40 min at the temperature of 90 DEG C to 100 DEG C at the speed of 250 r / min to 300 r / min, and the treated wastewater can be obtained after filtering. By means of the method, the sulfur content in the wastewater is greatly reduced; meanwhile, flocculating agents are greatly reduced, and the sulfur removing efficiency is improved.
Owner:何晓东
A method for enzymatically preparing 1,3-dioleic acid-2-palmitic acid triglyceride in a subcritical system
ActiveCN105219813BLower Diffusion LimitsImprove solubilityFermentationReaction rateTG - Triglyceride
Provided is a method for preparing 1,3-dioleoyl-2-palmitoylglycerol in a subcritical system, comprising the following steps: (1) preparation of raw materials: selecting, as a raw material A, lard having a cholesterol content of less than 0.05% by mass, or selecting, as raw material A, restructured palm oil having an sn-2 palmitic acid content of not less than 60% by mass; and, selecting as a raw material B, at least one of oleic acid and an oleic acid ester; and (2) subcritical enzyme-catalyzed transesterification: in a subcritical system, enabling raw materials A and B to undergo selective enzymatic transesterification in a reaction solvent under the catalysis of lipase to obtain 1,3-dioleoyl-2-palmitoylglycerol. With a rapid reaction rate, high selectivity, minimal enzyme usage, and no solvent residue, this method is readily adapted to large-scale continuous production.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com