Prasugrel salt and preparation method thereof
A technology of rapamoxate compound and compound, which is applied in carboxylate preparation, organic chemistry, etc., can solve problems such as not given more detailed evaluation, and achieve low hygroscopicity, simple operation, and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
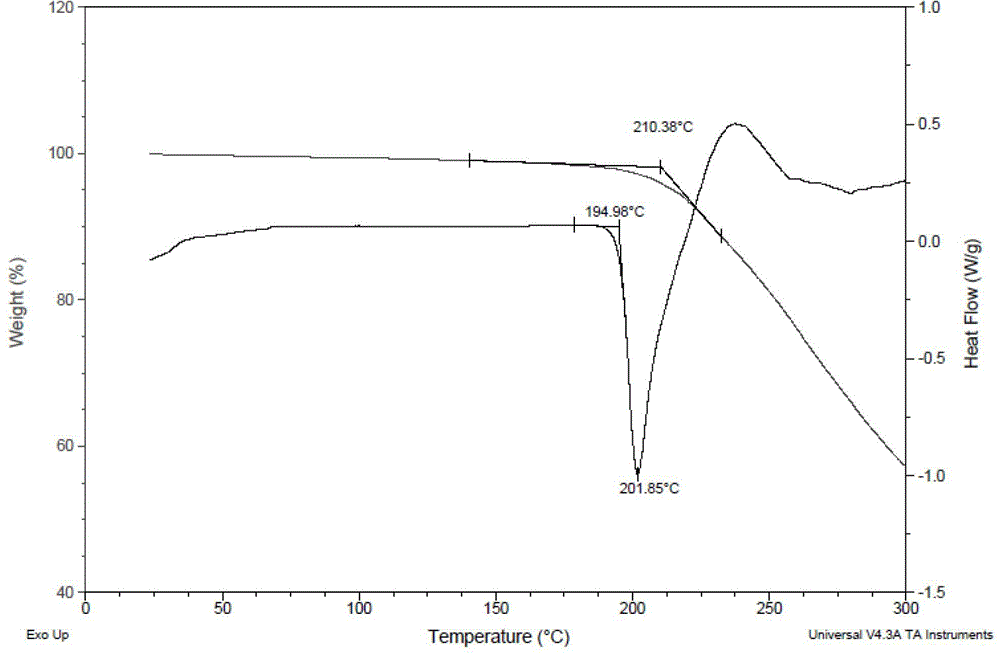
[0051] Dissolve 1.0 g of prasugrel free base in 20 ml of methanol, and add dropwise 100 ml of a methanol solution containing 1.04 g of pamoic acid. After dropping, stir at room temperature for 1 h to make the reaction complete. The reaction solution was left to stand at 5°C for 24 hours, filtered with suction, and vacuum-dried at 50°C for 2 hours to obtain 1.60 g of a light yellow solid with a melting point of 194.98°C.
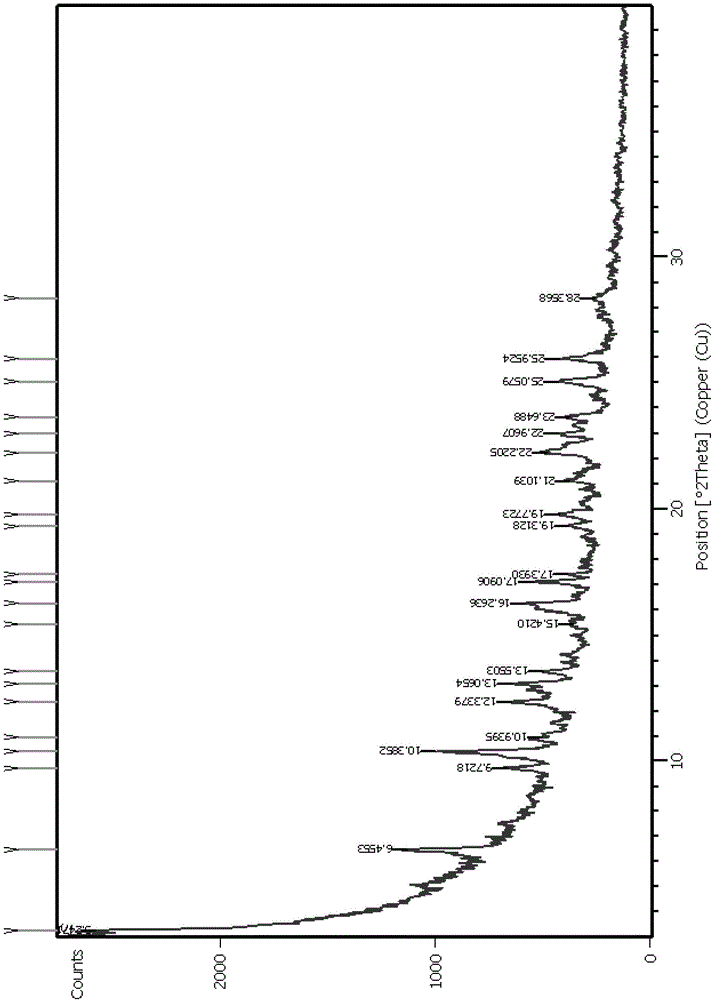
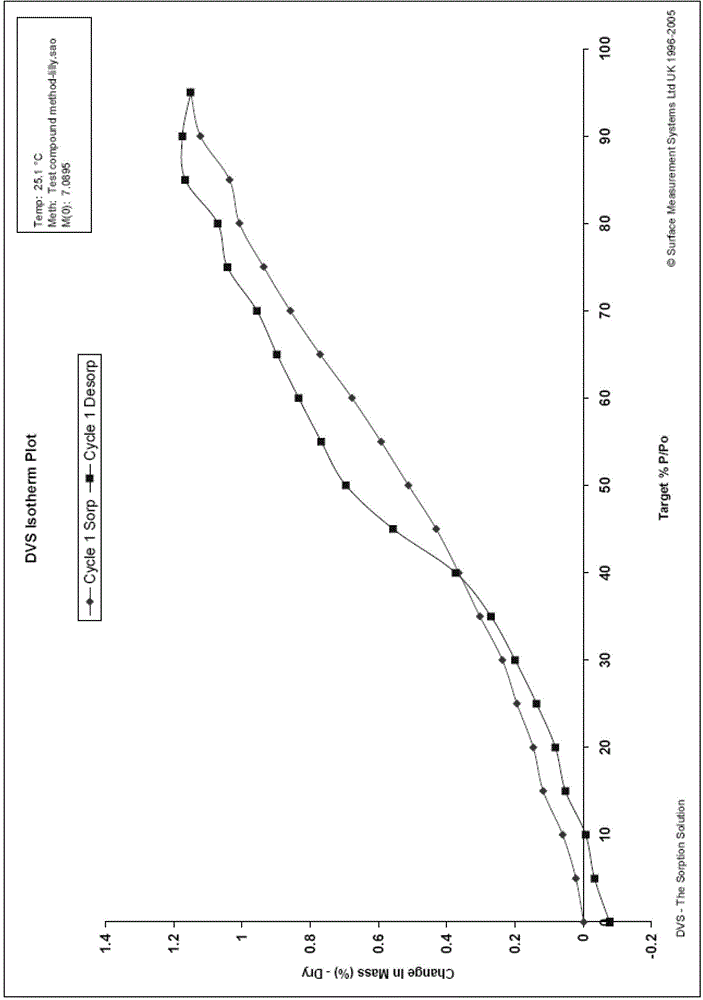
[0052] The resulting solid was subjected to X-ray powder diffraction (XRPD) analysis (see figure 1 ), DSC-TGA thermal analysis (see figure 2 ) and DVS dynamic moisture sorption analysis (see image 3 ), infrared spectroscopy (see Figure 4 ).
Embodiment 2
[0054] Dissolve 5.0 g of prasugrel free base in 50 ml of acetone, and add dropwise 600 ml of acetone solution containing 5.20 g of pamoic acid. After dropping, stir at room temperature for 1 h to make the reaction complete. The reaction solution was allowed to stand at 5°C for 24 hours, filtered with suction, and vacuum-dried at 50°C for 2 hours to obtain 7.85 g of a light yellow solid.
Embodiment 3
[0056] Dissolve 1.0 g of prasugrel free base in 15 ml of THF, and add dropwise 100 ml of a THF solution containing 1.04 g of pamoic acid. After dropping, stir at room temperature for 1 h to make the reaction complete. The reaction solution was left to stand at 5°C for 24 hours, filtered with suction, and dried in vacuo at 50°C for 2 hours to obtain 1.20 g of a light yellow solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com