A kind of liposome drug carrier and its preparation method and application
A liposome and pharmaceutical preparation technology, applied in the field of liposome drug carrier and its preparation, can solve the problems of poor liposome stability, liposome instability, and low encapsulation efficiency, and achieve uniform particle size , Improve the ability to enter the cell, good effect of encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Preparation of phospholipid liposomes loaded with bisphosphonates
[0081] 1.1 Preparation of reagents and stock solutions
[0082] Chloroform (analytical grade);
[0083] Phosphate buffer: Take 12.2mM disodium hydrogen phosphate and 8.2g / l NaCl in ultrapure water, adjust the pH to 7.40;
[0084] Stock solution: 100mg / ml EPC / HSPC, take 100mg EPC / HSPC and dissolve in chloroform;
[0085] 20mg / ml Chol, take 20mg Chol and dissolve in chloroform;
[0086] Dissolve an appropriate amount of bisphosphonate solution in ultrapure water, and adjust to pH 7.4 with 5N NaOH.
[0087] 1.2 Preparation method - thin film dispersion method
[0088] a) Take the stock solution egg yolk lecithin EPC or hydrogenated soybean lecithin HSPC and cholesterol in an eggplant-shaped bottle in a molar ratio of 2:1;
[0089] b) Slowly blow dry with nitrogen gas or evaporate chloroform with low-pressure rotation in a water bath to form a thin film in the eggplant-shaped bottle;
[0090...
Embodiment 2
[0096] Example 2 Preparation of Palmitic Acid and Cholesterol Liposome Loaded Bisphosphonates
[0097] 2.1 Preparation of reagents and stock solutions
[0098] Chloroform (analytical grade);
[0099] tert-butanol (analytical grade);
[0100] Phosphate buffer: take 12.2mM disodium hydrogen phosphate and 8.2g / l NaCl in ultrapure water, adjust the pH to 7.40;
[0101] Stock solution: 100mg / ml PA, take 100mgPA and dissolve in chloroform or tert-butanol;
[0102] 20mg / ml Chol, take 20mg Chol and dissolve in chloroform;
[0103] Dissolve an appropriate amount of bisphosphonate solution in ultrapure water, and adjust the pH to 7.60 with 5N NaOH.
[0104] 2.2 Preparation method 1 - thin film dispersion method
[0105] a) Take the stock solution palmitic acid PA and cholesterol in eggplant-shaped bottles in molar ratios of 3:5, 3:7, and 3:8;
[0106] b) Slowly blow dry with nitrogen gas or evaporate chloroform with low-pressure rotation in a water bath to form a thin film in the ...
Embodiment 3
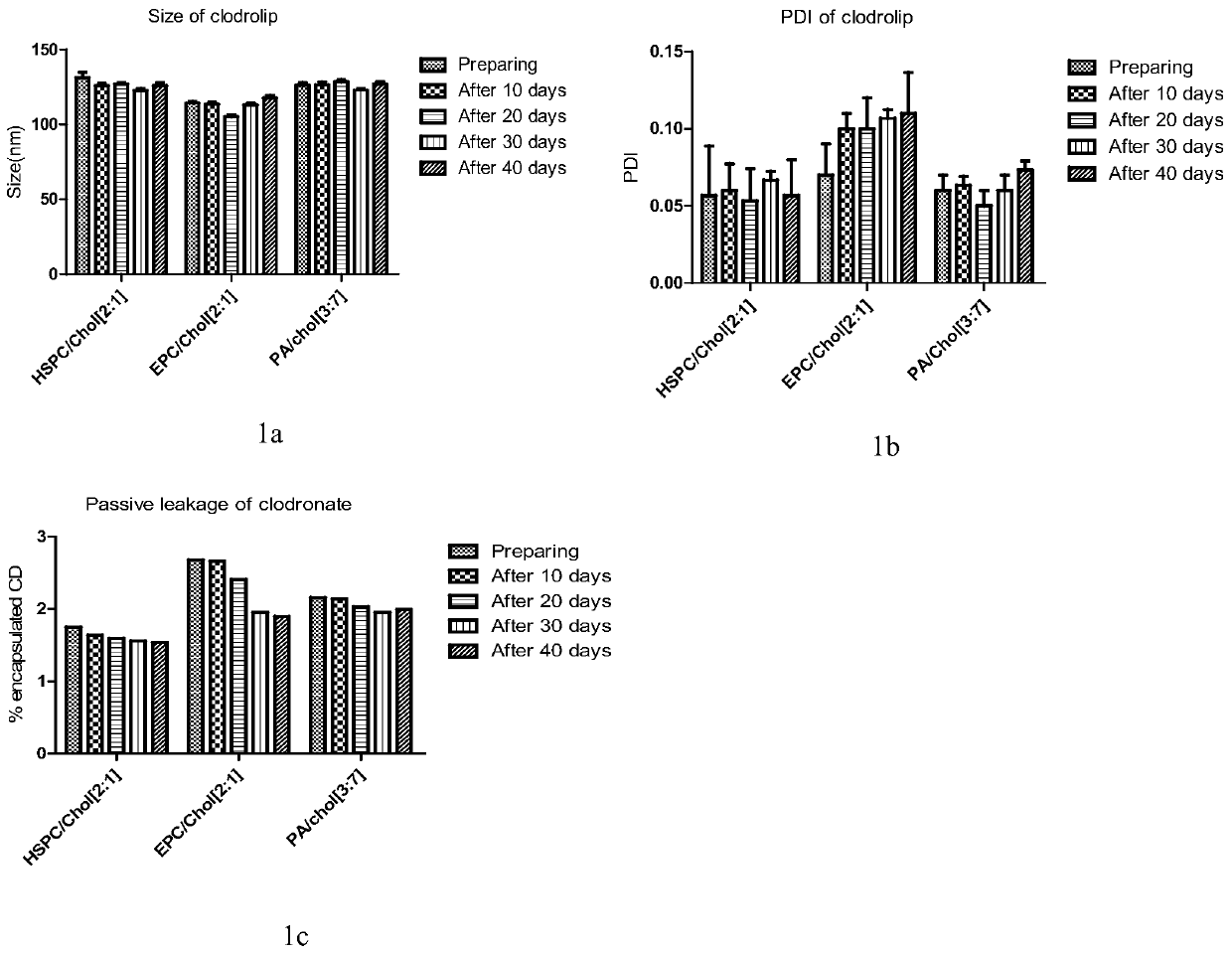
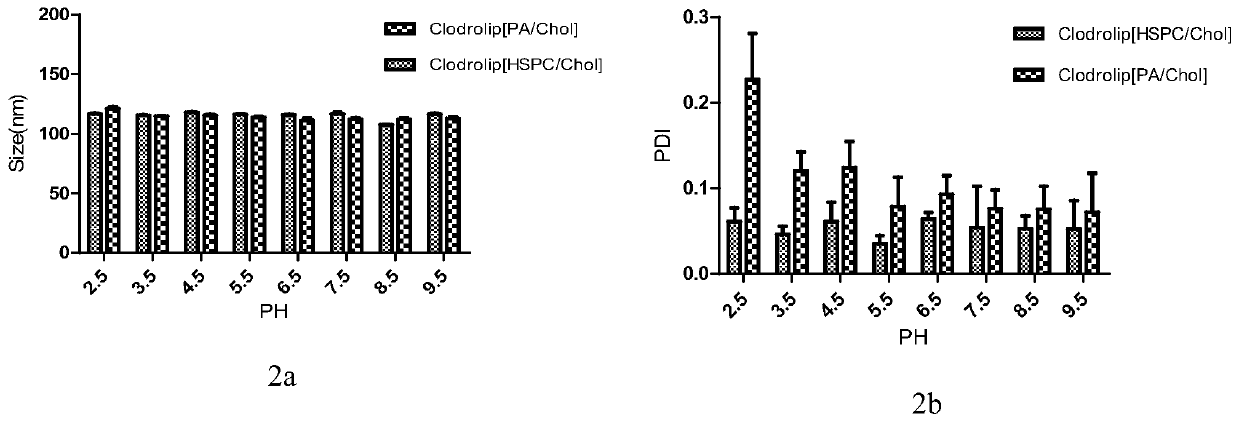
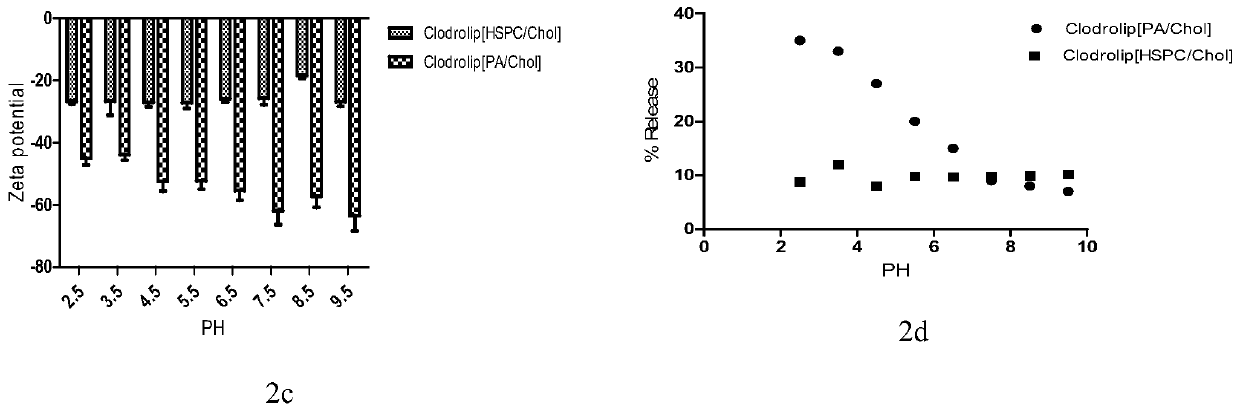
[0118] Example 3 Evaluation and comparison of the above-mentioned liposome-loaded bisphosphonate drugs
[0119] 3.1 Particle size, PDI, potentiometric determination
[0120] Instrument: dynamic light scattering laser particle size analyzer and zeta potential analyzer (zetasizer3000);
[0121] Conditions: The refractive index of the sample is set to that of silica (1.59), the dispersed phase is water (refractive index 1.36, viscosity 0.8cp), the detection temperature is 25 degrees, and the sample is equilibrated in the temperature of the detection chamber for 60 seconds before detection. The laser wavelength is 633nm, and the refraction angle is 90°;
[0122] Particle size and PDI measurement: 50 μl of sample was taken, diluted 10 times with pure water, transferred to the particle size cell, and each sample was measured in parallel three times.
[0123] Potentiometric measurement: 50 μl of sample was taken, diluted 10 times with 10% Hepes solution, transferred to a potential ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com