Stearic acid-modified cell penetrating peptide and its preparation and application
A technology of stearic acid and penetrating peptide, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and polypeptides containing positioning/targeting motifs, etc. Polypeptide structure, permeability limitations and other problems, to improve the ability to enter cells, overcome the inability to enter cells, and achieve the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
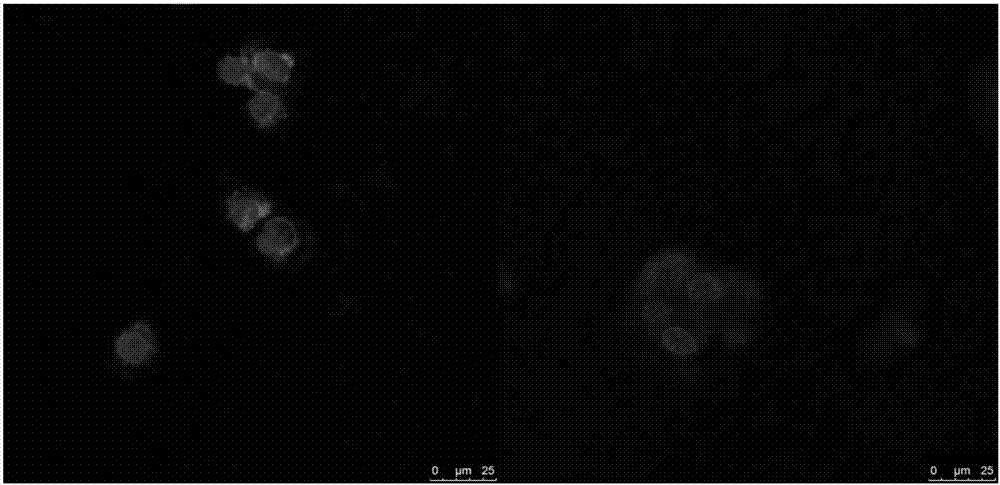
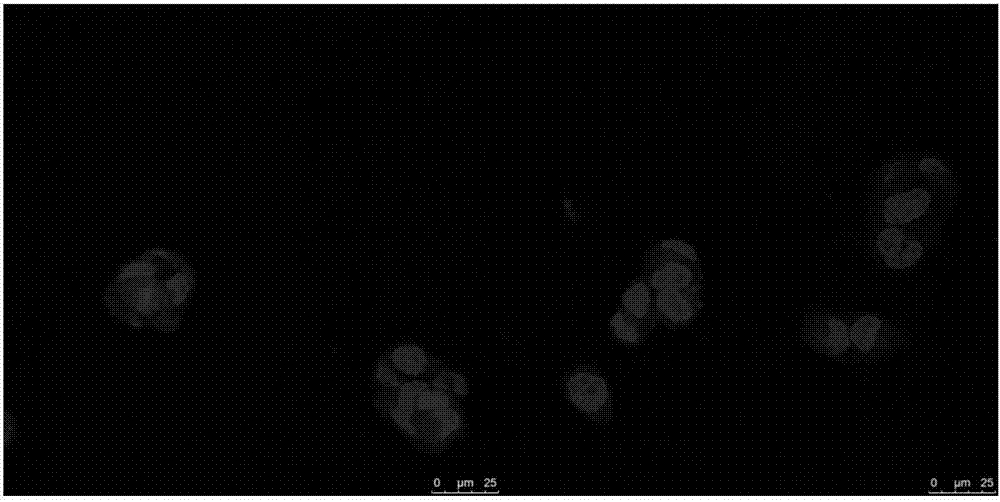
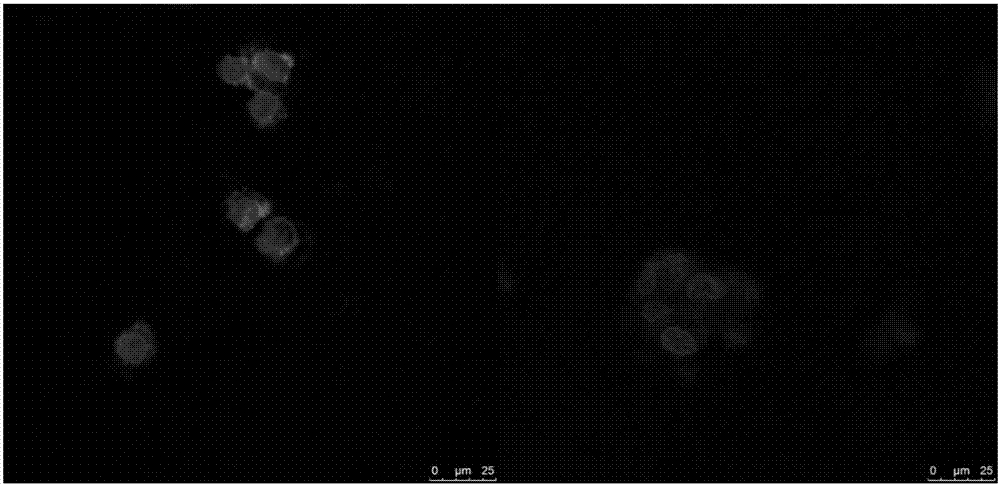
Image
Examples
Embodiment 1
[0055] Example 1 Preparation and acquisition of cell penetrating peptide CPP-SA
[0056] 1.1 The polypeptide CPP is prepared and obtained by chemical synthesis, the amino acid sequence of the CPP is shown in SEQ ID NO.1, specifically: Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Lys (GRKKRRQRRRK). Then stearic acid is modified on the C-terminal Lys of the CPP, specifically, the carboxyl group on the C-terminal Lys of the CPP can be amidated, and then the carboxyl group of stearic acid and the C-terminal Lys of the CPP can be The amino group of the side chain was subjected to condensation reaction to prepare a cell-penetrating peptide whose C-terminus of CPP was modified with stearic acid, which was named CPP-SA. After verification and characterization, the structural formula of CPP-SA is correct, as shown in formula (1), specifically:
[0057] 1.2 Prepare and obtain the polypeptide CPP-MMP by chemical synthesis, the amino acid sequence of the CPP-MMP is shown in SEQ ID NO.2, sp...
Embodiment 2
[0062] Embodiment 2 fusion peptide Gaegurins5-CPP-SA and liposome preparation thereof
[0063] (1) The C-terminus of Gaegurins5 is connected to the N-terminus of CPP-SA by chemical synthesis method to prepare and obtain the fusion peptide Gaegurins5-CPP-SA. The amino sequence of Gaegurins5 is shown in SEQ ID NO.3, specifically: Phe-Leu-Gly-Ala-Leu-Phe-Lys-Val-Ala-Ser-Lys-Val-Leu-Pro-Ser-Val-Lys- Cys-Ala-Ile-Thr-Lys-Lys-Cys.
[0064] (2) Surfactant dialysis method prepares liposome preparation:
[0065] a) Preparation of liposome suspension: get each liposome component HSPC, Chol according to the ratio, mix well, N 2 Blow dry to obtain a lipid film, add 5% glucose, ultrasonically hydrate at 40°C with a power of 80% for 30 min, and pass through an 80 nm film 21 times with an Extruder to obtain a liposome suspension;
[0066] b) Preparation of fusion peptide surfactant solution: take fusion peptide Gaegurins5-CPP-SA and surfactant OG according to the ratio, and mix them unifor...
Embodiment 3
[0118] Embodiment 3 fusion peptide HNPs1-CPP-SA and liposome preparation thereof
[0119] (1) The C-terminus of human neutrophil peptide-1 (HNPs1) was connected to the N-terminus of CPP-SA by chemical synthesis method to prepare and obtain the fusion peptide HNPs1-CPP-SA. The amino sequence of HNPs1 is shown in SEQ ID NO.4, specifically: Ala-Cys-Tyr-Cys-Arg-Ile-Pro-Ala-Cys-Ile-Ala-Gly-Glu-Arg-Arg-Tyr-Gly- Thr-Cys-Ile-Tyr-Gln-Gly-Arg-Leu-Trp-Ala-Phe-Cys-Cys.
[0120] (2) Surfactant dialysis method prepares liposome preparation:
[0121] a) Preparation of liposome suspension: get each liposome component EPC, Chol by proportioning, mix well, N 2 Blow dry to obtain a lipid film, add 5% glucose, ultrasonically hydrate at 40°C with a power of 80% for 30 min, and pass through an 80 nm film 21 times with an Extruder to obtain a liposome suspension;
[0122] b) Preparation of fusion peptide surfactant solution: take fusion peptide HNPs1-CPP-SA and surfactant n-nonyl-β-D-glucopyranos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com