Solid cefprozi lipid nanoparticle preparation
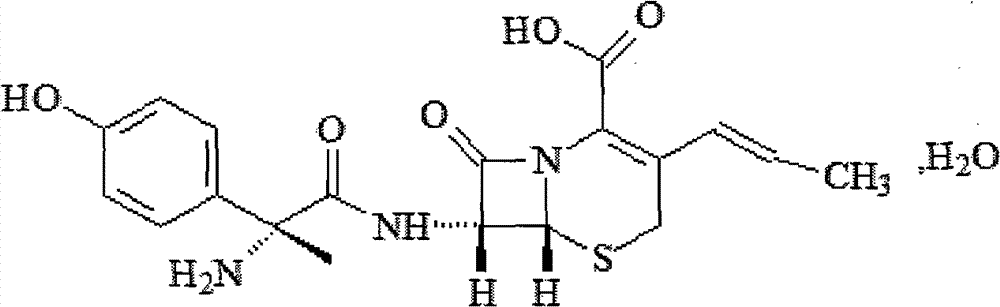
A cefprozil, solid lipid nanotechnology, applied in the field of medicine, to achieve the effects of increasing concentration, improving solubility, and improving controlled release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
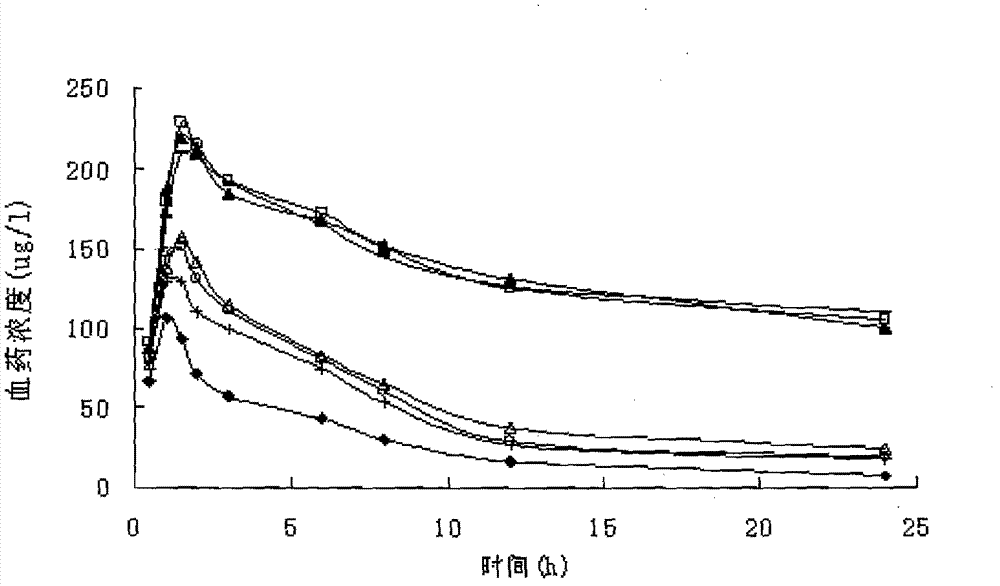
Image
Examples
preparation example Construction
[0074] On the other hand, the present invention provides the preparation method of cefprozil solid lipid nanoparticle, the method comprises the following steps:
[0075] (a) adding palmitic acid and glyceryl tristearate into an organic solvent, heating in a constant temperature water bath at 50° C., stirring to dissolve it completely, then adding cefprozil, fully stirring to dissolve it, and forming an organic phase;
[0076] (b) dissolving polyoxyethylene 40 stearate in water, heating in a constant temperature water bath at 50°C, and stirring to dissolve it to form a water phase;
[0077] (c) Slowly add the organic phase into the stirring water phase, keep the temperature at 50° C., and continue stirring for 1 h;
[0078] (d) removing the organic solvent under reduced pressure to obtain translucent colostrum;
[0079] (e) the colostrum in the above steps is quickly added to an appropriate amount of cold water under the stirring condition of 2000 rpm, and the high-pressure ho...
Embodiment 1
[0134] The preparation of embodiment 1 cefprozil solid lipid nanoparticle sheet
[0135] The raw materials used are as follows:
[0136]
[0137] Adopt following production process to prepare cefprozil solid lipid nanoparticle sheet:
[0138] (1) 150g of palmitic acid and 75g of glyceryl tristearate are added into 750ml volume ratio in the mixed solvent of chloroform and methyl alcohol of 5: 1, 50 ℃ of constant temperature water baths are heated, and stirring makes it dissolve completely, then adds 125g cefprozil, Thoroughly stir to make it dissolve and form the organic phase;
[0139] (2) Dissolve 100g of polyoxyethylene 40 stearate in 2000ml of water, heat in a constant temperature water bath at 50°C, stir to dissolve, and form a water phase;
[0140] (3) Slowly add the organic phase into the stirring water phase, keep the temperature at 50° C., and continue stirring for 1 h;
[0141] (4) remove organic solvent under reduced pressure, obtain translucent colostrum;
...
Embodiment 2
[0147] The preparation of embodiment 2 cefprozil solid lipid nanoparticle capsules
[0148] The raw materials used are as follows:
[0149]
[0150] Adopt following production process to prepare cefprozil solid lipid nanoparticle capsules:
[0151] (1) 200g of palmitic acid and 100g of glyceryl tristearate are added into 1000ml of a volume ratio of 5: 1 in the mixed solvent of chloroform and methanol, heated in a constant temperature water bath at 50°C, stirred to make it dissolve completely, then add 250g of cefprozil, Thoroughly stir to make it dissolve and form the organic phase;
[0152] (2) Dissolve 80g of polyoxyethylene 40 stearate in 400ml of water, heat in a constant temperature water bath at 50°C, stir to dissolve, and form a water phase;
[0153](3) Slowly add the organic phase into the stirring water phase, keep the temperature at 50° C., and continue stirring for 1 h;
[0154] (4) remove organic solvent under reduced pressure, obtain translucent colostrum;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com