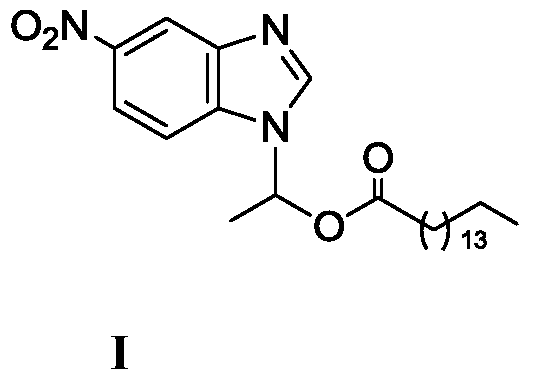
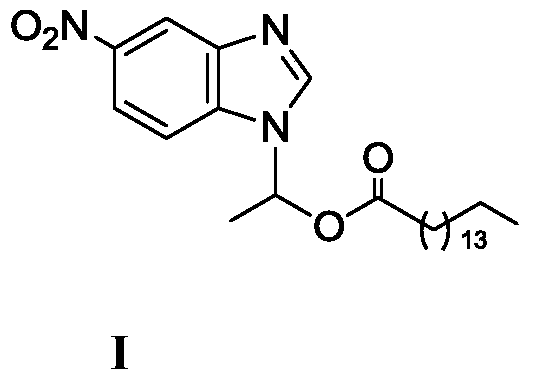
A kind of lipase-catalyzed method for synthesizing 1-(6-nitrobenzimidazolyl) ethyl palmitate on-line
A technology of nitrobenzimidazole and vinyl palmitate is applied in the field of lipase-catalyzed online synthesis of 1-ethyl palmitate, which can solve the problems of low conversion rate and selectivity, long reaction time and the like, and shorten the reaction time. Time, high conversion rate, the effect of reducing the reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of 1-(6-nitrobenzimidazolyl) ethyl palmitate
[0033]
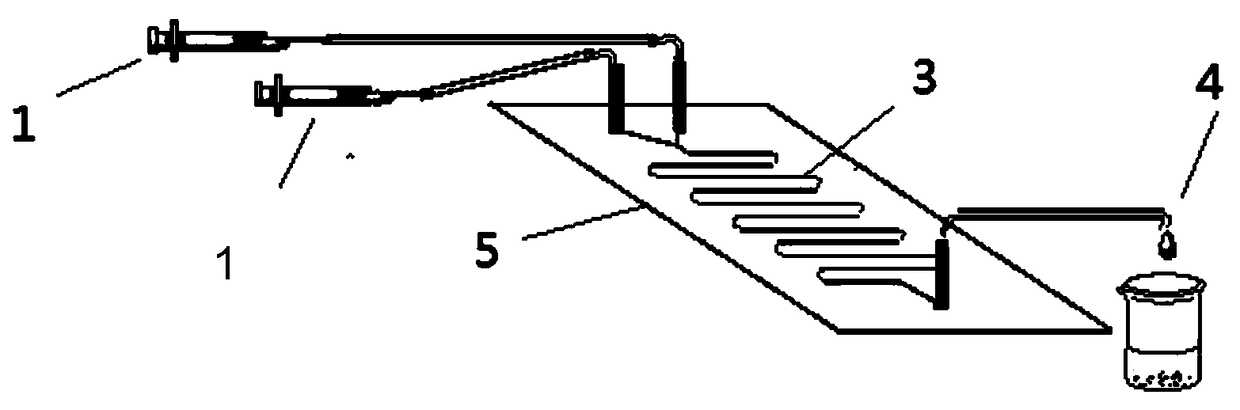
[0034] device reference figure 1 : 6-nitro-benzimidazole (1.0mmol) was dissolved in 10mL DMSO, vinyl palmitate (6.0mmol) was dissolved in 10mL DMSO, and then filled in two 10mL syringes for use. 0.87g of lipase LipozymeTLIM was evenly filled in the reaction channel, driven by the PHD2000 syringe pump, the two reaction solutions were separated at 10.4 μL min -1 The flow rate enters the reaction channel through the Y-shaped interface for reaction, and the temperature of the reactor is controlled at 50°C through a water bath thermostat. The reaction solution flows continuously in the reaction channel for 30 minutes, and the reaction results are tracked and detected by thin-layer chromatography (TLC).
[0035] The reaction solution was collected online by the product collector, and the solvent was distilled off under reduced pressure to obtain the crude product, which was wet-packed with...
Embodiment 2-4
[0040] Change the temperature of the microfluidic channel reactor, others are the same as in Example 1, and the reaction results are as shown in Table 1:
[0041] Table 1: Effect of Temperature on Reaction
[0042]
[0043]
[0044] The results in Table 1 show that when the flow rate is 10.4 μL min -1 , when the reaction time is 30min, the conversion rate increases significantly with the increase of the reaction temperature. When the reaction temperature reaches 50°C, the conversion rate of the reaction is the best. If the temperature continues to rise at this time, the enzyme activity will decrease. , thereby causing the conversion rate of the reaction to decrease, so the optimal reaction temperature of 1-(6-nitrobenzimidazolyl)ethyl palmitate in the microfluidic microchannel reactor of the present invention is 50°C.
Embodiment 5-8
[0046] Change the substrate molar ratio of vinyl palmitate and 6-nitro-benzimidazole in the microfluidic microchannel reactor to be 1:1 (embodiment 5), 2:1 (embodiment 6), 4:1 ( Embodiment 7), 8:1 (embodiment 8), the consumption 1.0mmol of 6-nitro-benzimidazole is constant, changes the consumption of vinyl palmitate into 1.0mmol (embodiment 5), 2.0mmol ( Example 6), 4.0mmol (Example 7), 8.0mmol (Example 8), were dissolved in 10mLDMSO respectively, and others were the same as in Example 1. The results are shown in Table 2.
[0047] Table 2: Effect of 6-nitro-benzimidazole and vinyl palmitate substrate molar ratio on reaction
[0048]
[0049] The result of table 2 shows, along with the increase of reactant vinyl palmitate, the conversion rate of reaction also increases thereupon, and when substrate ratio is 6:1, the conversion rate of reaction is optimum, and 6-nitro-benzo Essentially 72% of the imidazole has been converted to 1-(6-nitrobenzimidazolyl)ethyl palmitate. Now ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com