Lurasidone pamoate amorphous substance as well as preparation method and application thereof
A technology of lurasidone pamoic acid and lurasidone hydrochloride, which is applied in the field of amorphous pamoic acid lurasidone and its preparation, can solve the problems of low oral bioavailability, low solubility, poor water solubility, etc. , to improve oral bioavailability, improve solubility and avoid irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
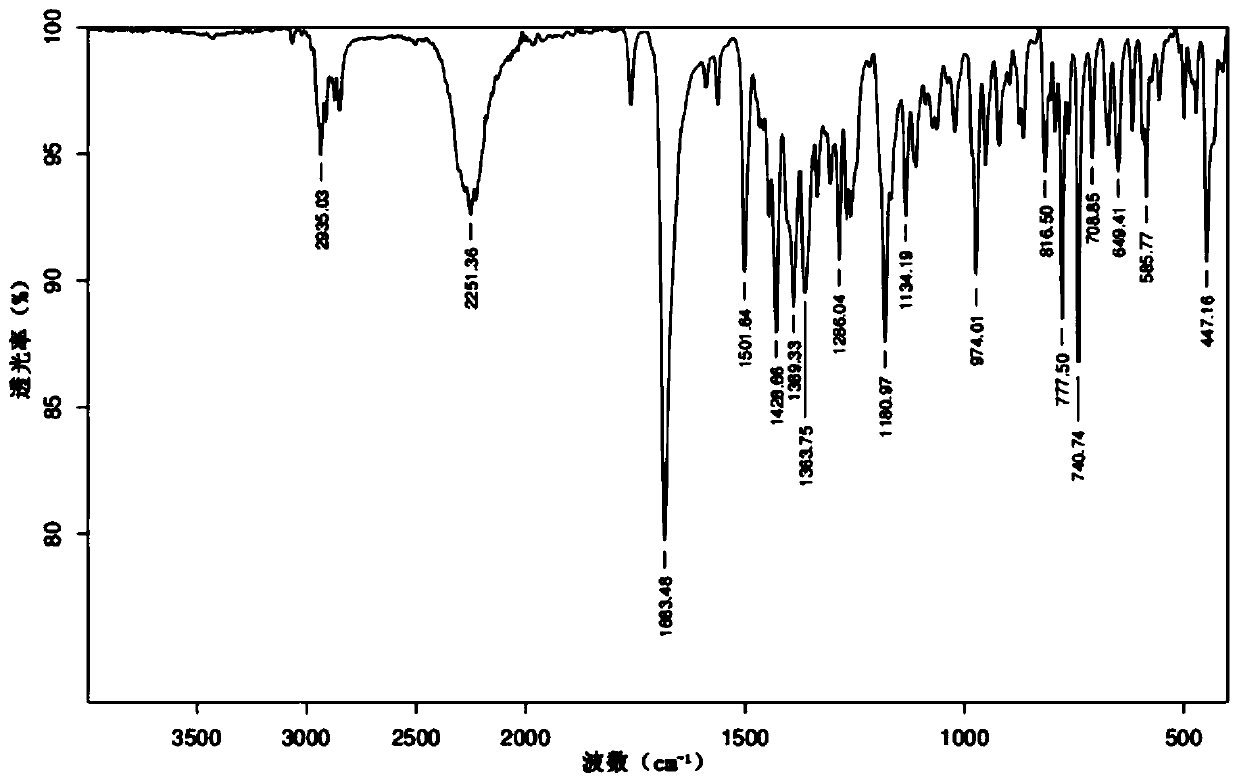
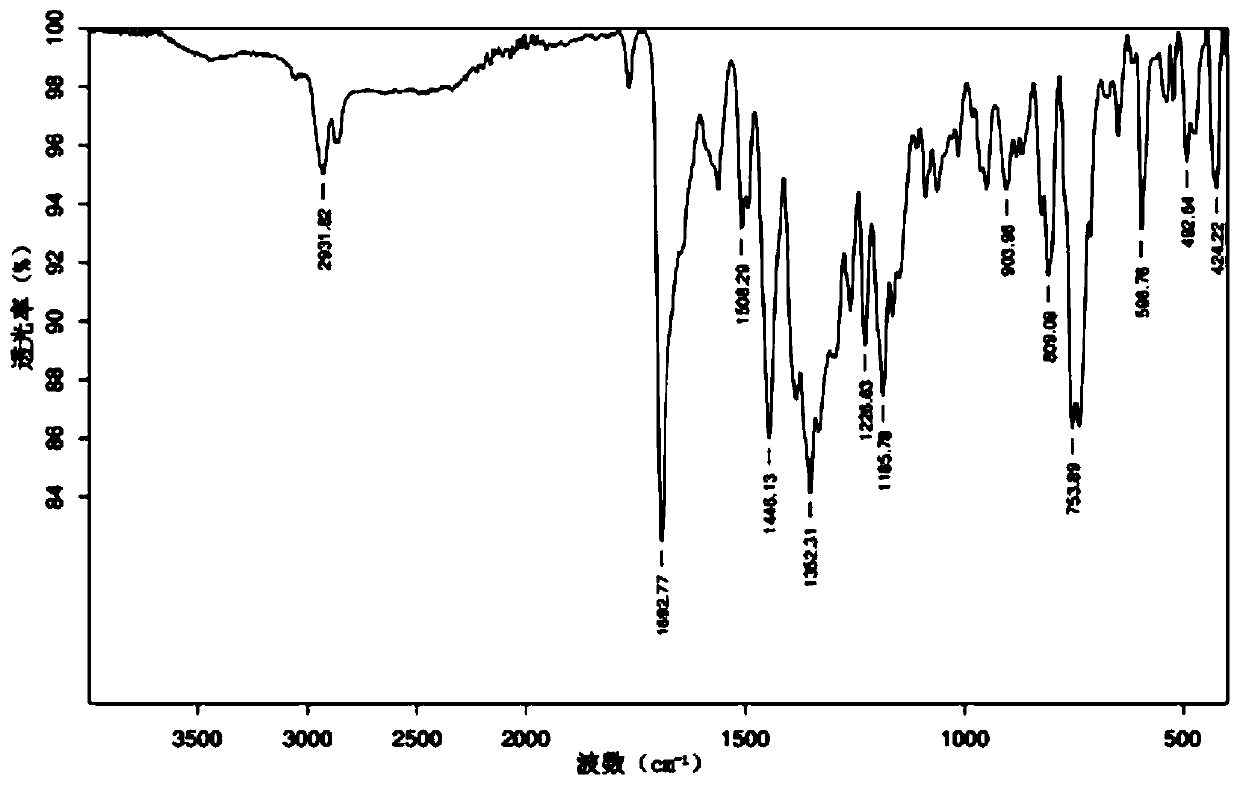
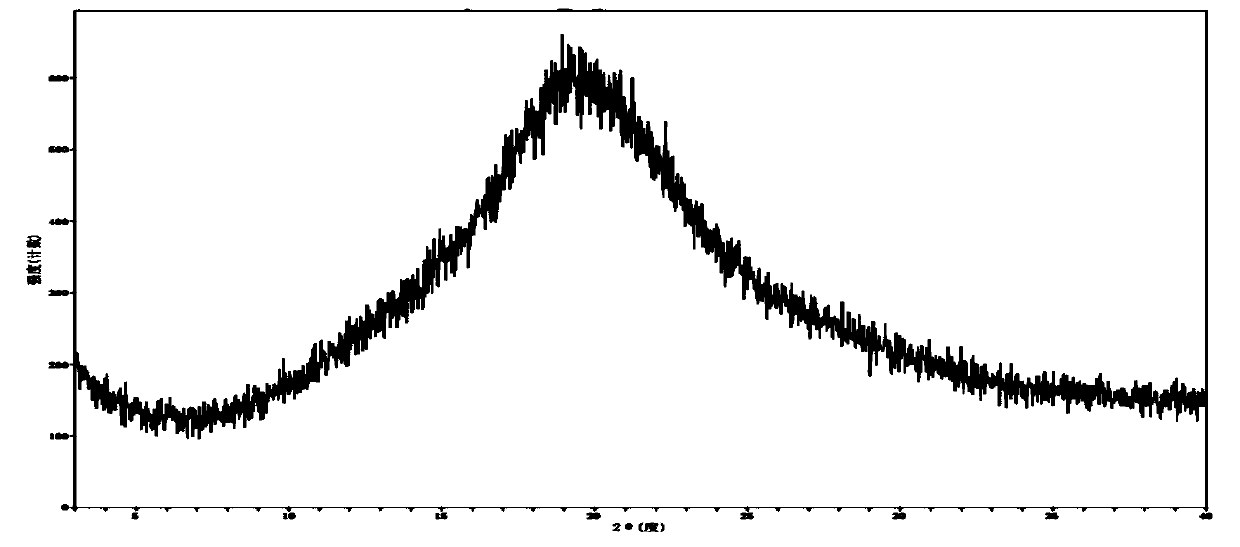
Image
Examples
preparation example Construction
[0041] The second aspect of the present invention provides a method for preparing pamoic acid lurasidone amorphous, the method comprising the following steps:
[0042] (1) dissolving lurasidone hydrochloride and pamoic acid disodium salt, or lurasidone and pamoic acid in tetrahydrofuran to obtain a first mixed solution;
[0043] (2) stirring the first mixed solution at 55-60° C. for 2-3 hours to react to obtain a second mixed solution;
[0044] (3) The second mixed solution was rotary evaporated at 55-60° C. until the solvent was spin-dried, and then water was used as the precipitation solvent for precipitation to obtain lurasidone pamoic acid amorphous substance.
[0045] In the method of the present invention, in step (1), the molar ratio of lurasidone hydrochloride and pamoic acid disodium salt is 1:2-2.5; Specifically, for example, it can be 1:2, 1: 2.1, 1:2.2, 1:2.3, 1:2.4 or 1:2.5; preferably, in step (1), the molar ratio of lurasidone hydrochloride to pamoic acid disod...
Embodiment 1
[0058] Dissolve lurasidone hydrochloride and pamoic acid disodium salt with a molar ratio of 1:2 in 20ml of tetrahydrofuran, stir and react at 60°C for 2h, then spin evaporate to dry the solvent. Add 10ml of water for precipitation, and filter to obtain the first amorphous product A1 after precipitation of crystals.
Embodiment 2
[0060] Lurasidone and pamoic acid with a molar ratio of 1:1 were dissolved in 20ml of tetrahydrofuran, stirred and reacted at 55°C for 3h, and then evaporated to dry the solvent. Add 10ml of water for precipitation, and filter to obtain the second amorphous product A2 after precipitation of crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com