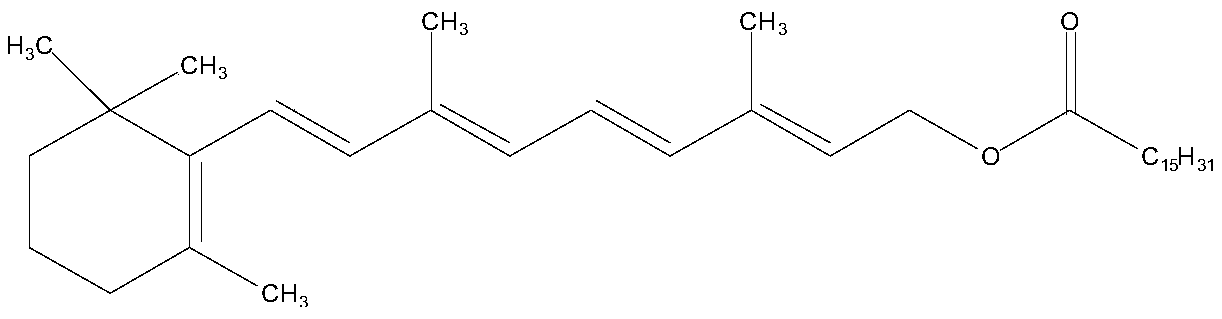
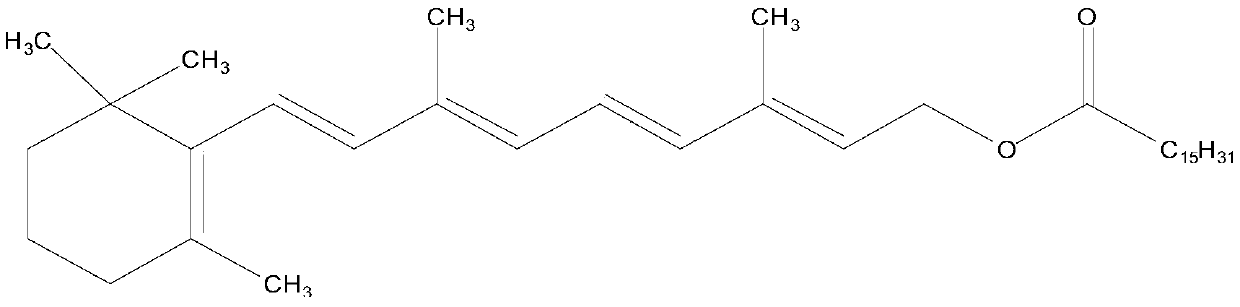
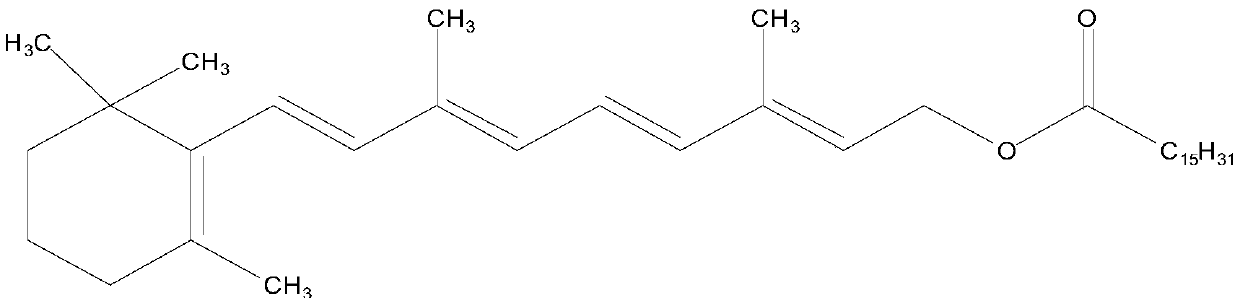
Enzyme-catalyzed method for preparing vitamin A palmitate
A technology for the preparation of palmitate and catalysis, applied in the direction of fermentation, etc., can solve the problems of lower alcohol residue, high cost, cumbersome steps, etc., and achieves the effects of mild reaction conditions, good product quality, and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Vitamin A acetate (10g, purity 95.35%, 29.03mmol) and methyl palmitate (8.23g, 30.48mmol) were added in cyclohexane (50ml), stirred for 30min, after fully dissolving, lipase Novozyme 435 was added ( 50 g), stirred, vacuumized, and the temperature of the enzyme-catalyzed transesterification reaction was controlled by adjusting the vacuum to be 25 ° C. At the same time, the reaction product methyl paraacetate and a small amount of cyclohexane were separated through the rectification column. After reacting for 10 hours, the feed liquid was filtered. Filter out lipase, add alumina (15g) to the filtrate, stir for 30min, filter to remove alumina. The filtrate was evaporated under reduced pressure to recover n-hexane to obtain 15.19 g of light yellow oil, which was analyzed according to the method of USP28. The results showed that the purity of vitamin A palmitate was 1.7566 million IU / g, equivalent to a content of 96.62%. The pure yield is 96.34%.
Embodiment 2
[0036] Add vitamin A acetate (10g, purity 96.28%, 29.31mmol) and ethyl palmitate (9.17g, 32.24mmol) in n-heptane (100ml), stir for 30min, after fully dissolving, add lipase Novozyme 435 ( 90g), stirred, vacuumized, and the temperature of the enzyme-catalyzed transesterification reaction was controlled by adjusting the vacuum to be 30°C. At the same time, the reaction by-product ethyl acetate and a small amount of n-heptane were separated through the rectification column. After reacting for 8 hours, the feed liquid was filtered. Filter out lipase, add diatomaceous earth (30g) to the filtrate, stir for 30min, filter to remove diatomite. The filtrate was evaporated under reduced pressure to recover n-heptane to obtain 15.34 g of light yellow oil, which was analyzed according to the method of USP28. The results showed that the purity of vitamin A palmitate was 1.7622 million IU / g, equivalent to a content of 96.93%. The pure yield is 96.65%.
Embodiment 3
[0038] Add vitamin A acetate (10g, purity 95.68%, 29.13mmol) and isopropyl palmitate (10g, 33.50mmol) in n-octane (150ml), stir for 30min, after fully dissolving, add lipase Novozyme 435 ( 120g), stirred, vacuumized, and the temperature of the enzyme-catalyzed transesterification reaction was controlled to be 40°C by adjusting the vacuum, and the reaction by-product isopropyl acetate and a small amount of n-octane were separated through the rectification column. After reacting for 6 hours, the feed liquid was filtered. Filter out the lipase, add aluminum oxide (30 g) to the filtrate, stir for 30 min, and filter to remove the aluminum oxide. The filtrate was evaporated under reduced pressure to recover n-octane to obtain 15.21 g of light yellow oil, which was analyzed according to the method of USP28. The results showed that the purity of vitamin A palmitate was 1.7587 million IU / g, equivalent to a content of 96.73%. The pure yield is 96.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com