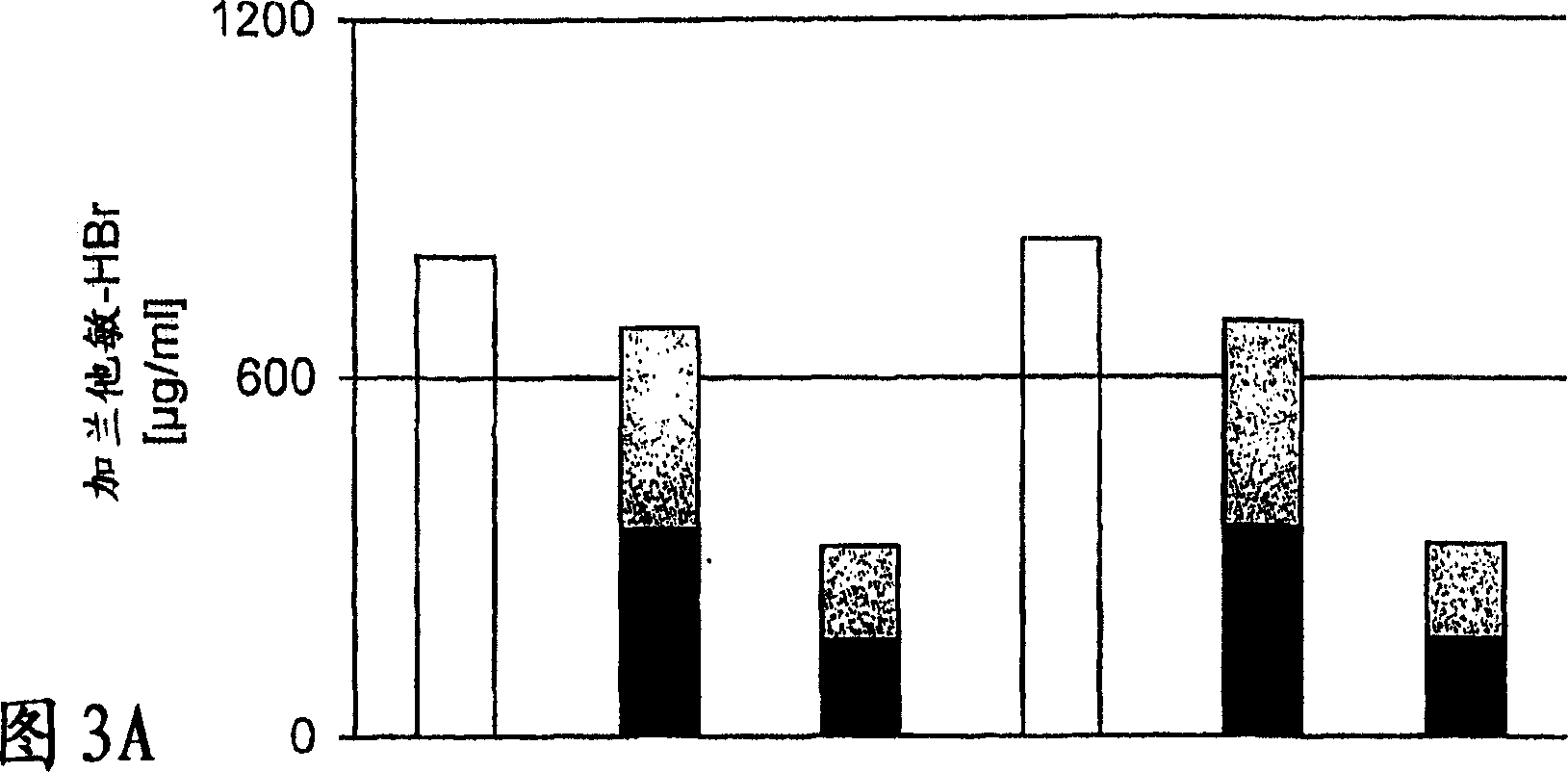
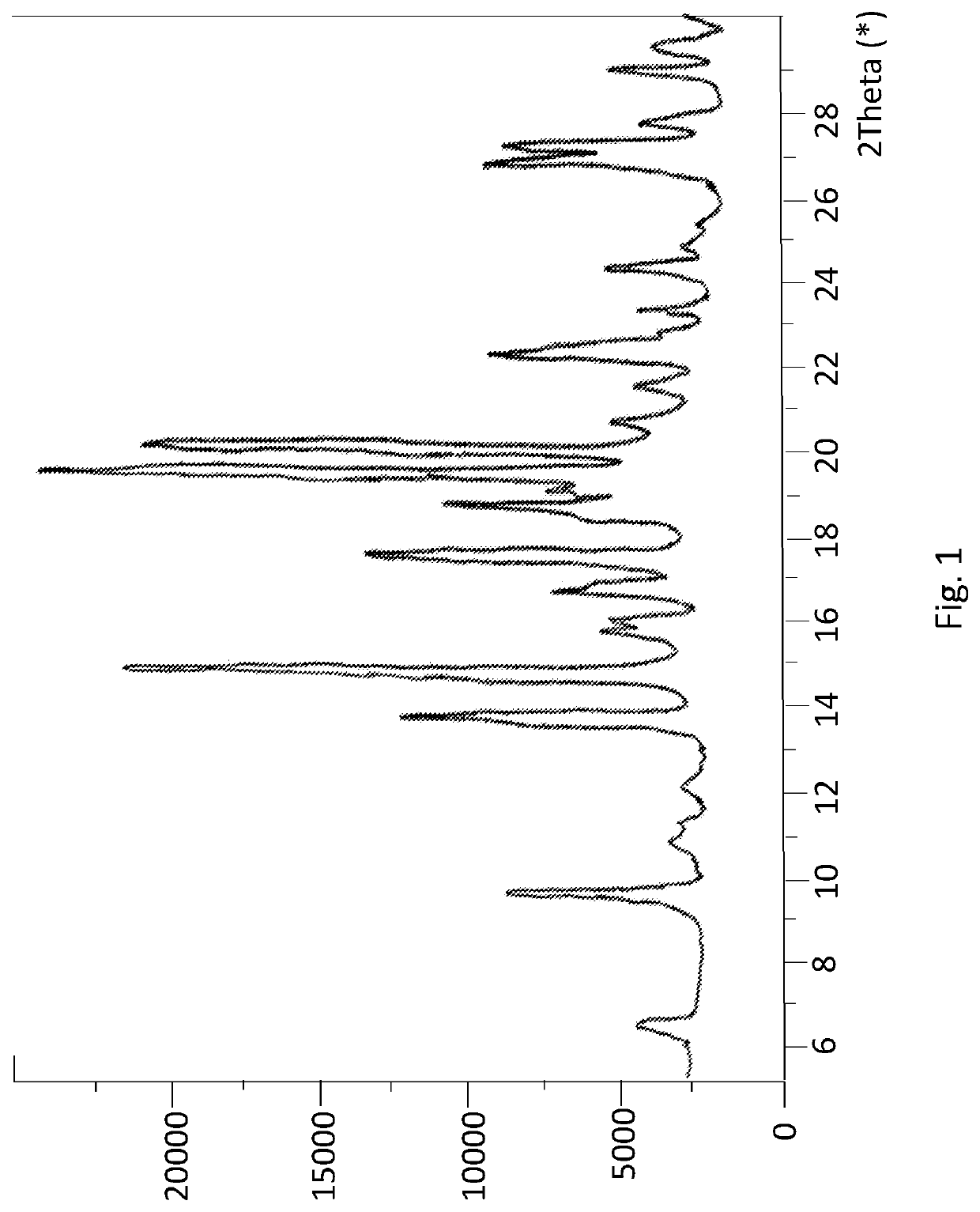
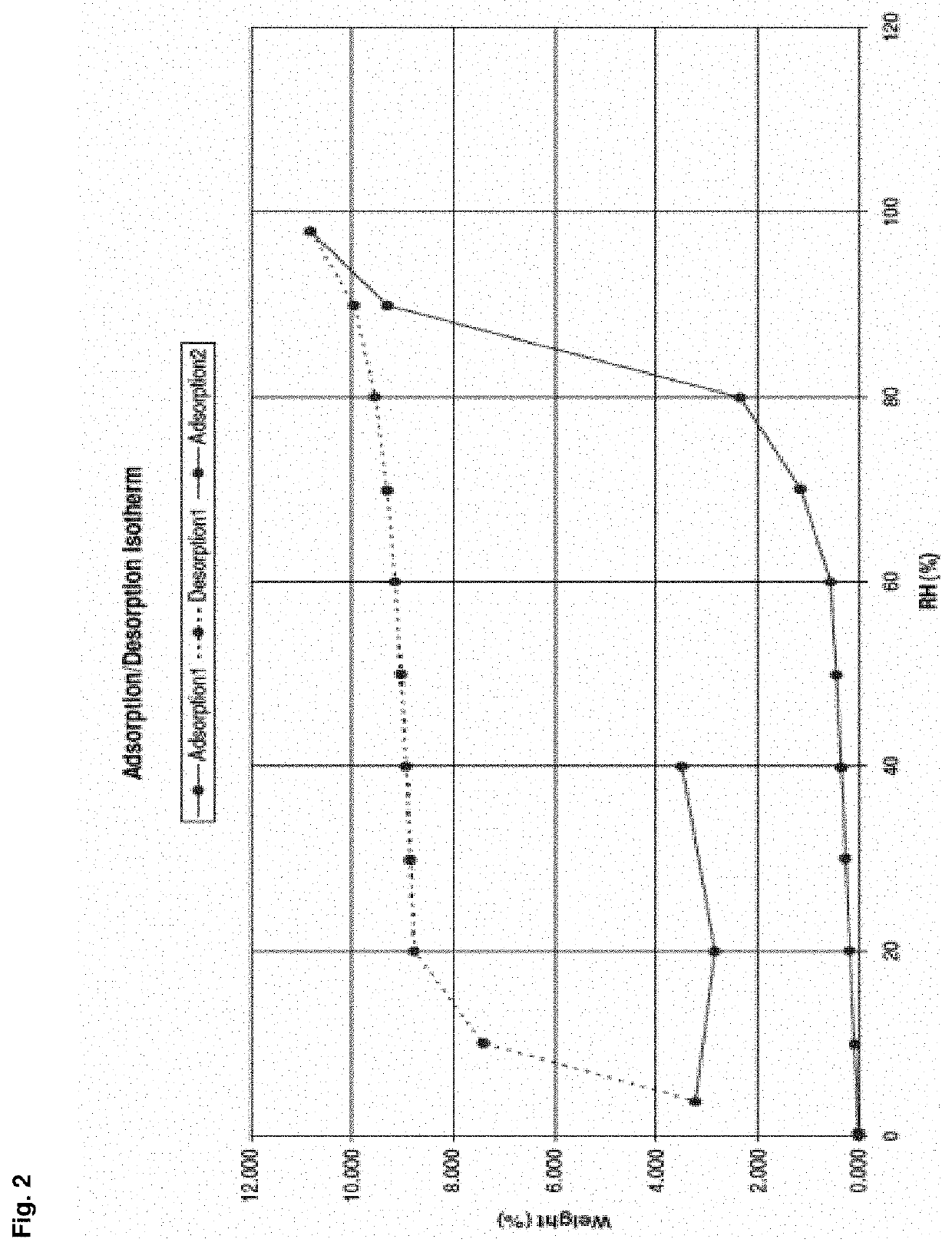
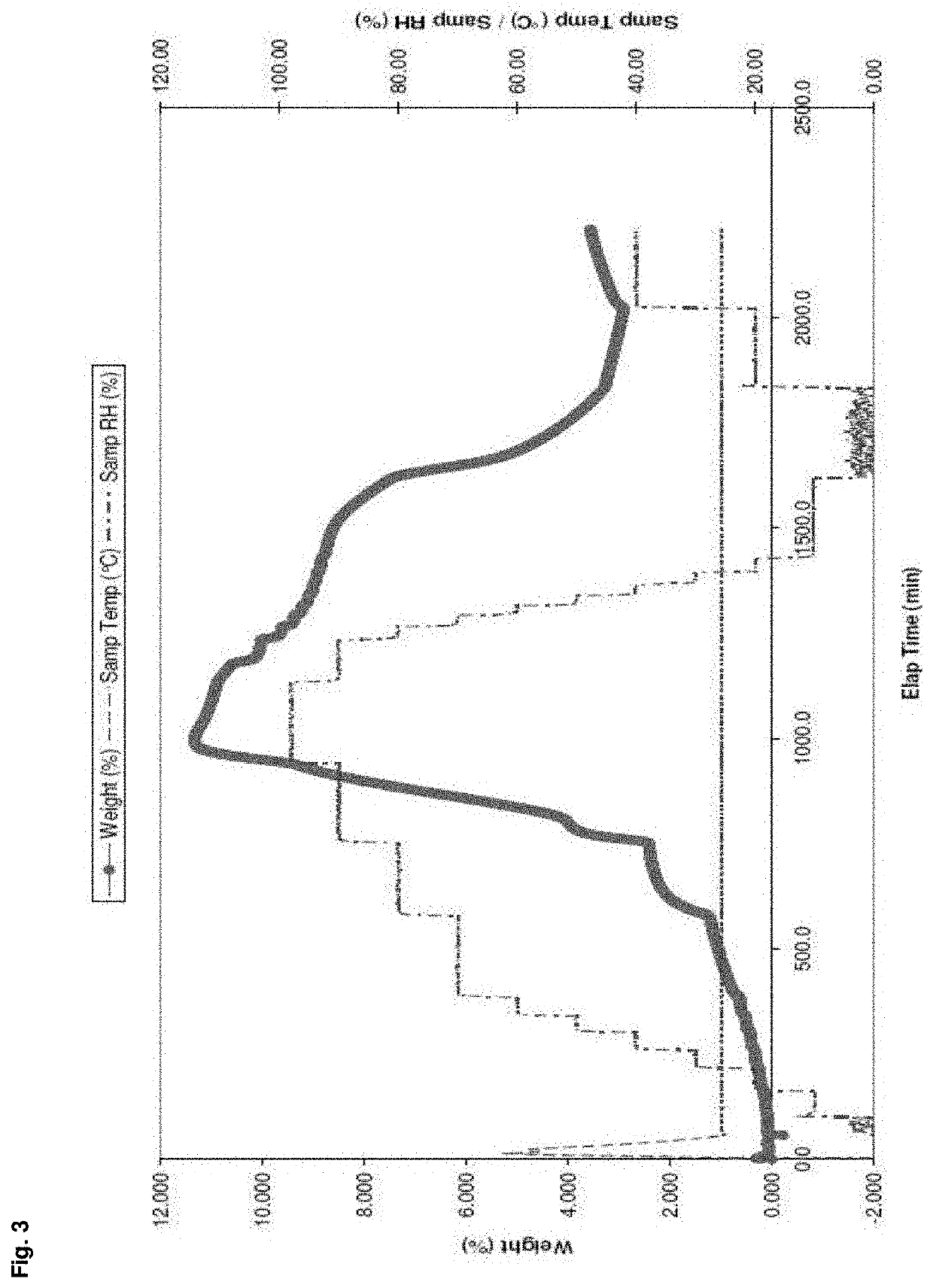
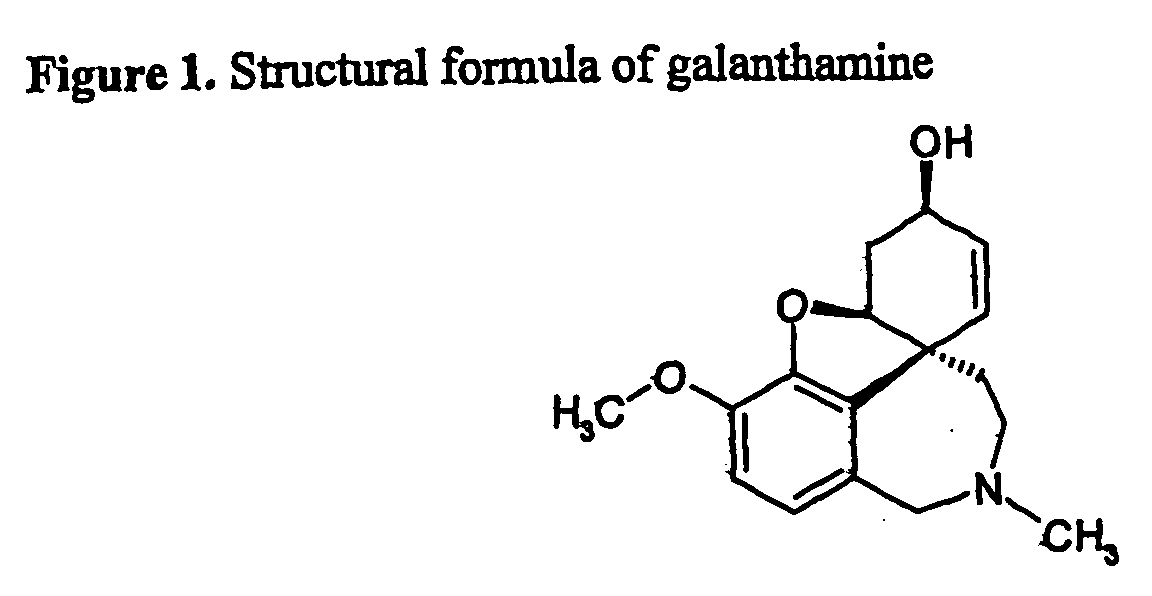
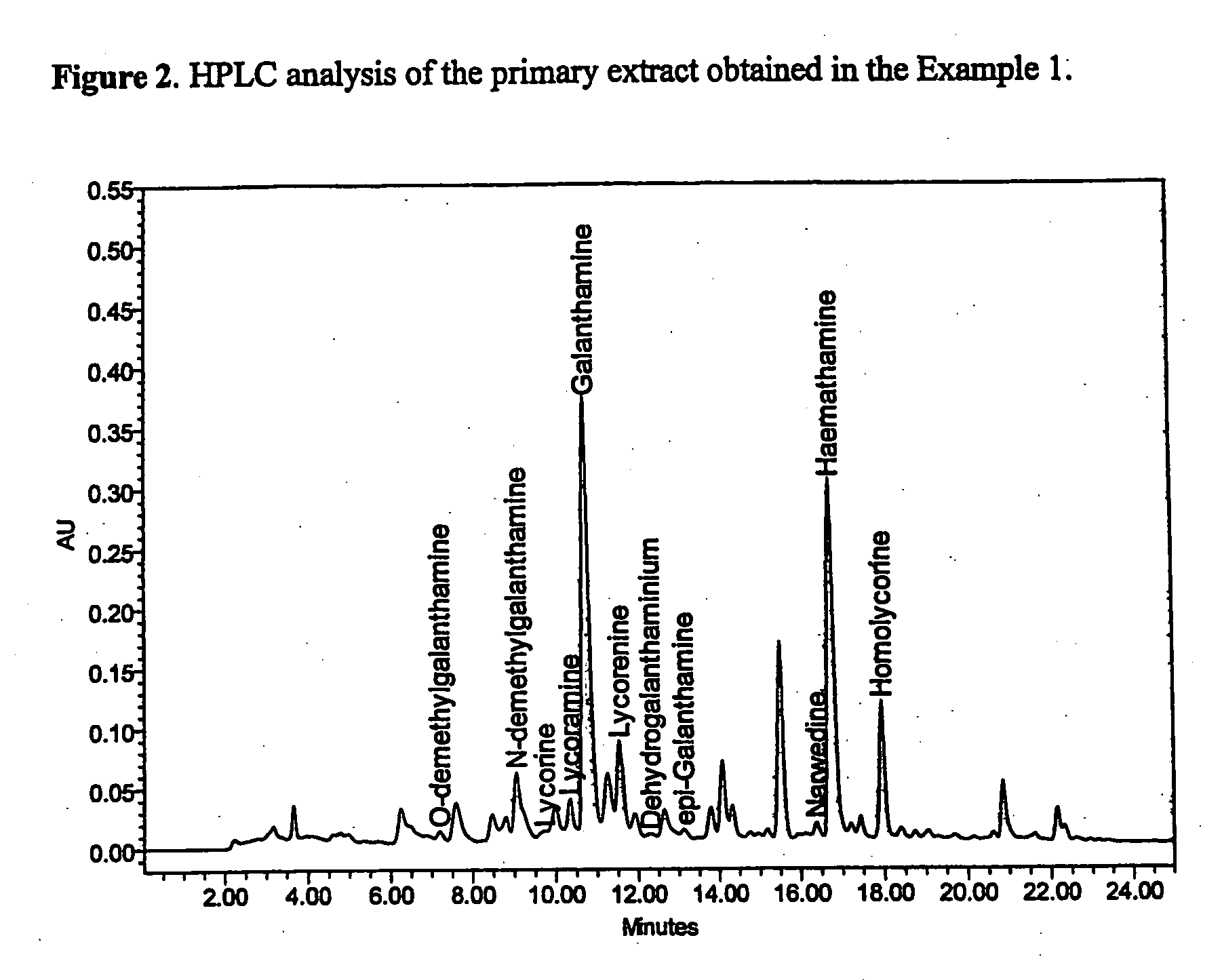
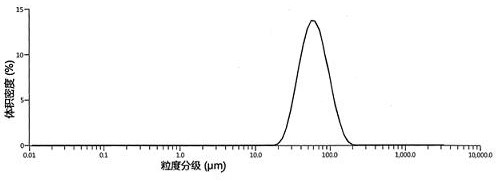
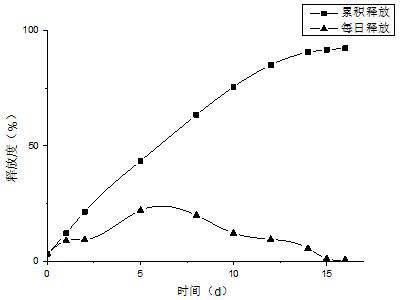
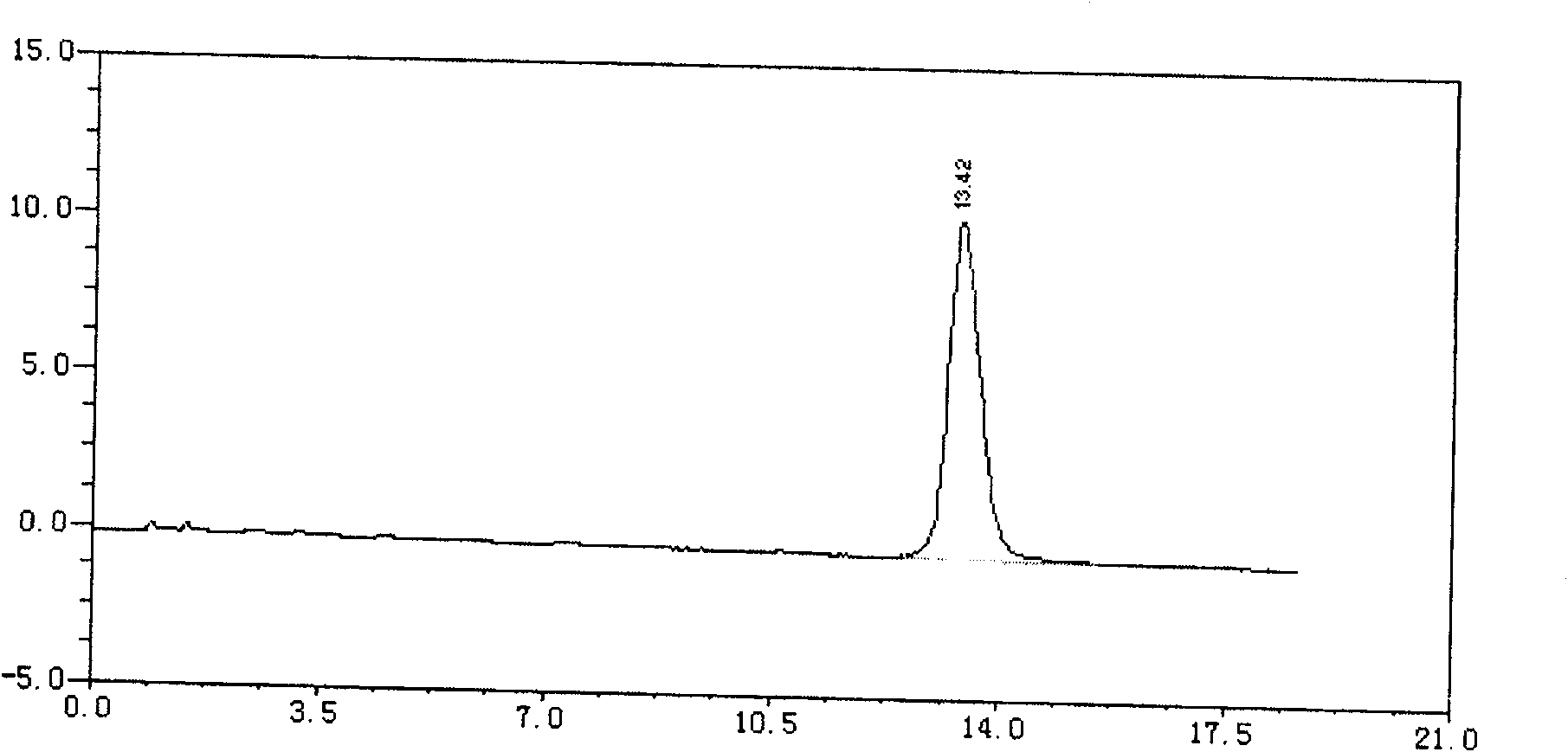
Patents
Literature
41 results about "Galantamin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
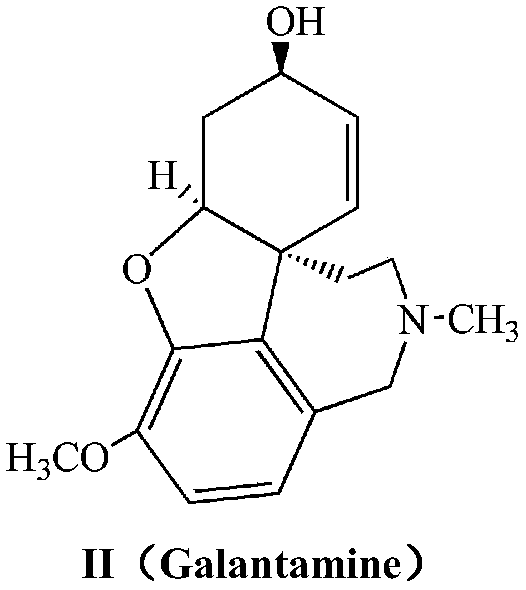
Cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment
InactiveUS20090253654A1Improve efficacyLow peripheral side effectBiocideGroup 5/15 element organic compoundsChemical structureChemical synthesis
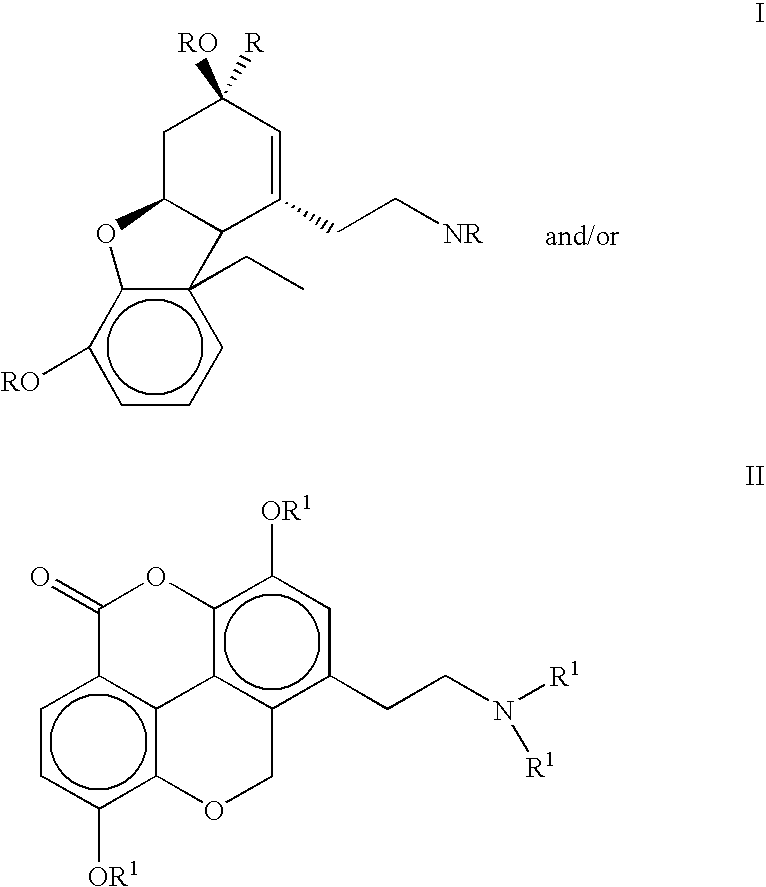
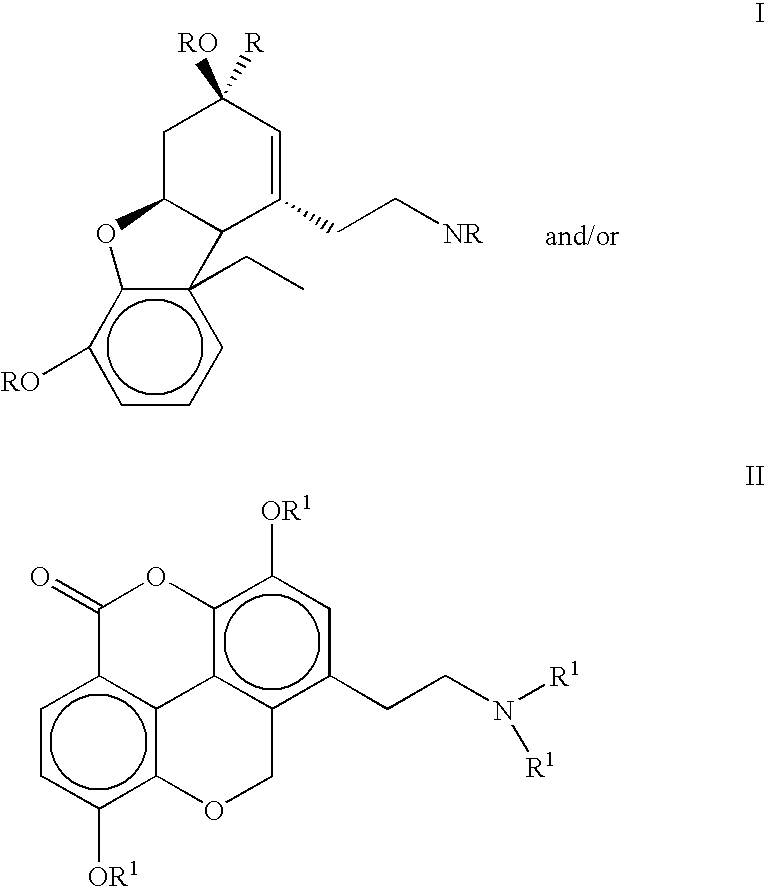
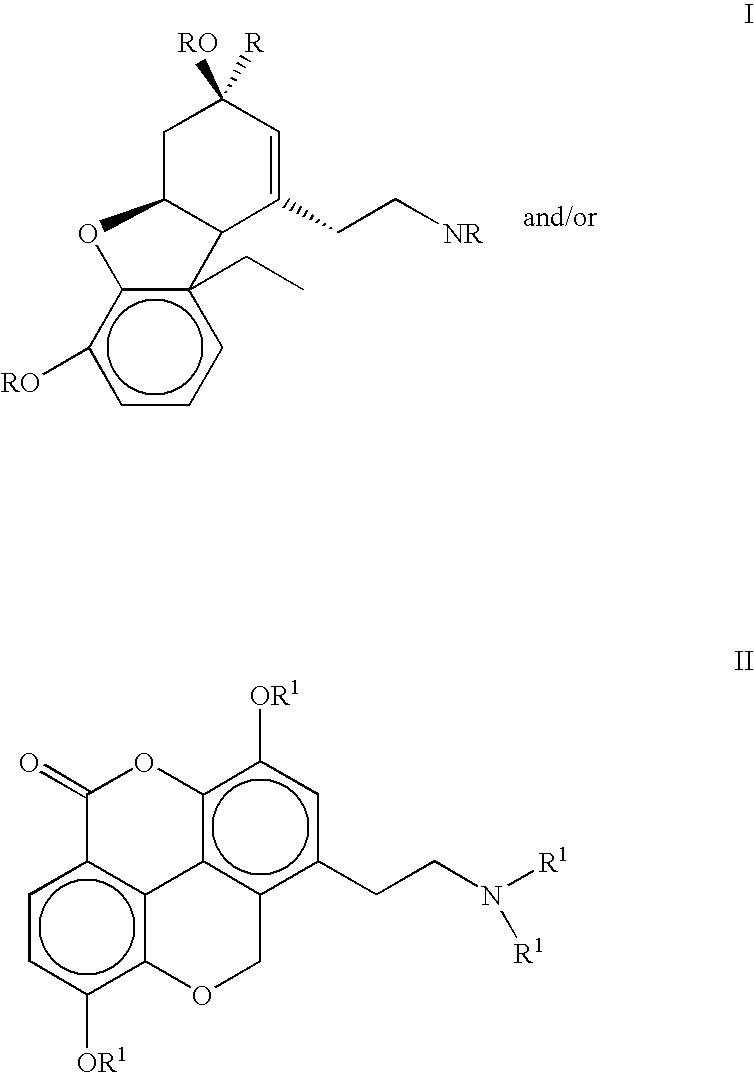
The present invention refers to compounds that, in addition to enhancing the sensitivity to acetylcholine and choline, and their exogenous agonists, of neuronal cholinergic receptors and / or acting as cholinesterase inhibitors and / or neuroprotective agents, have enhanced blood-brain barrier permeability in comparison to their parent compounds. The compounds are derived (either formally by their chemical structure or directly by chemical synthesis) from natural compounds belonging to the class of amaryllidaceae alkaloids e.g., galantamine, narwedine and lycoramine, or from metabolites of said compounds. The compounds of the present invention can either interact as such with their target molecules, or they can act as “pro-drugs”, in the sense that after reaching their target regions in the body they are converted by hydrolysis or enzymatic attack to the original parent compound and react as such with their target molecules, or both. The compounds of this invention may be used as medicaments.
Owner:GALANTOS PHARMA
Chiral synthesis method for (-)-galantamin hydrobromide
InactiveCN101239983AFavorable manufacturing methodNervous disorderAsymmetric synthesesHydrobromideEphedrine
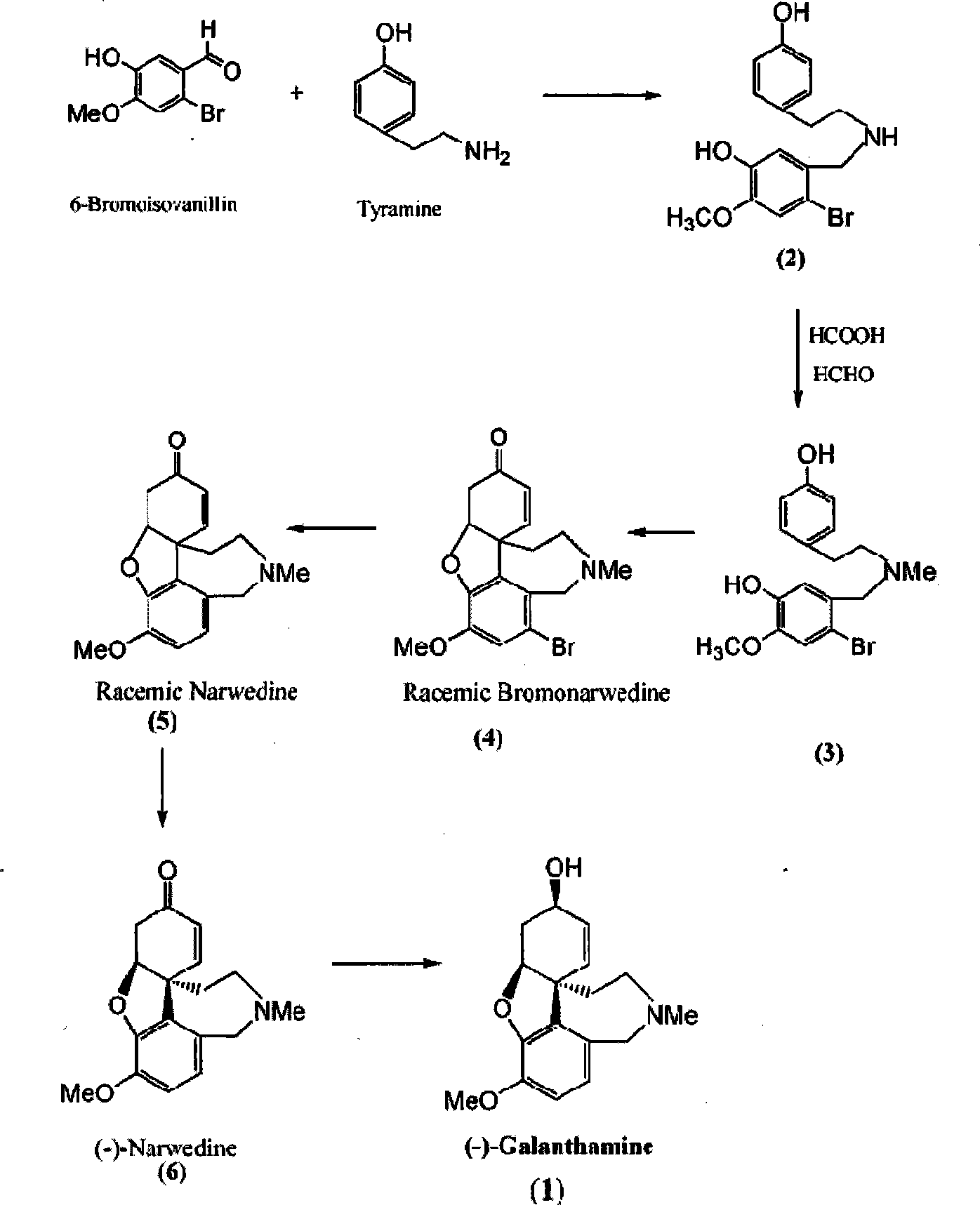
Ethamine compound is obtained by condensing starting materials of 6-bromoisovanillin and tyramine under catalysis of NaBH4 in carbinol. In formylation of ethamine compound, aminic acid and formaldehyde of same amount are used to produce formyl compound, with temperature between 90 DEG C. and 110 DEG C., 8h. Racemic bromonarwedine is obtain by oxidation and cyclization of the formyl compound. Racemic narwedine is obtained by reduction reaction under catalysis of NaCO2H, PPh3, Pd(OAc)2. Racemic narwedine is transformed into (-)-narwedine by a process of crystal inoculation. (-)-narwedine is reduced into unsaturated ketone by (-)-N-methyl ephedrine as chiral reagent, whereby optically active alcohol is obtained, and (-)-galanthamine is also obtained.
Owner:泰州市宝嵘新材料有限公司
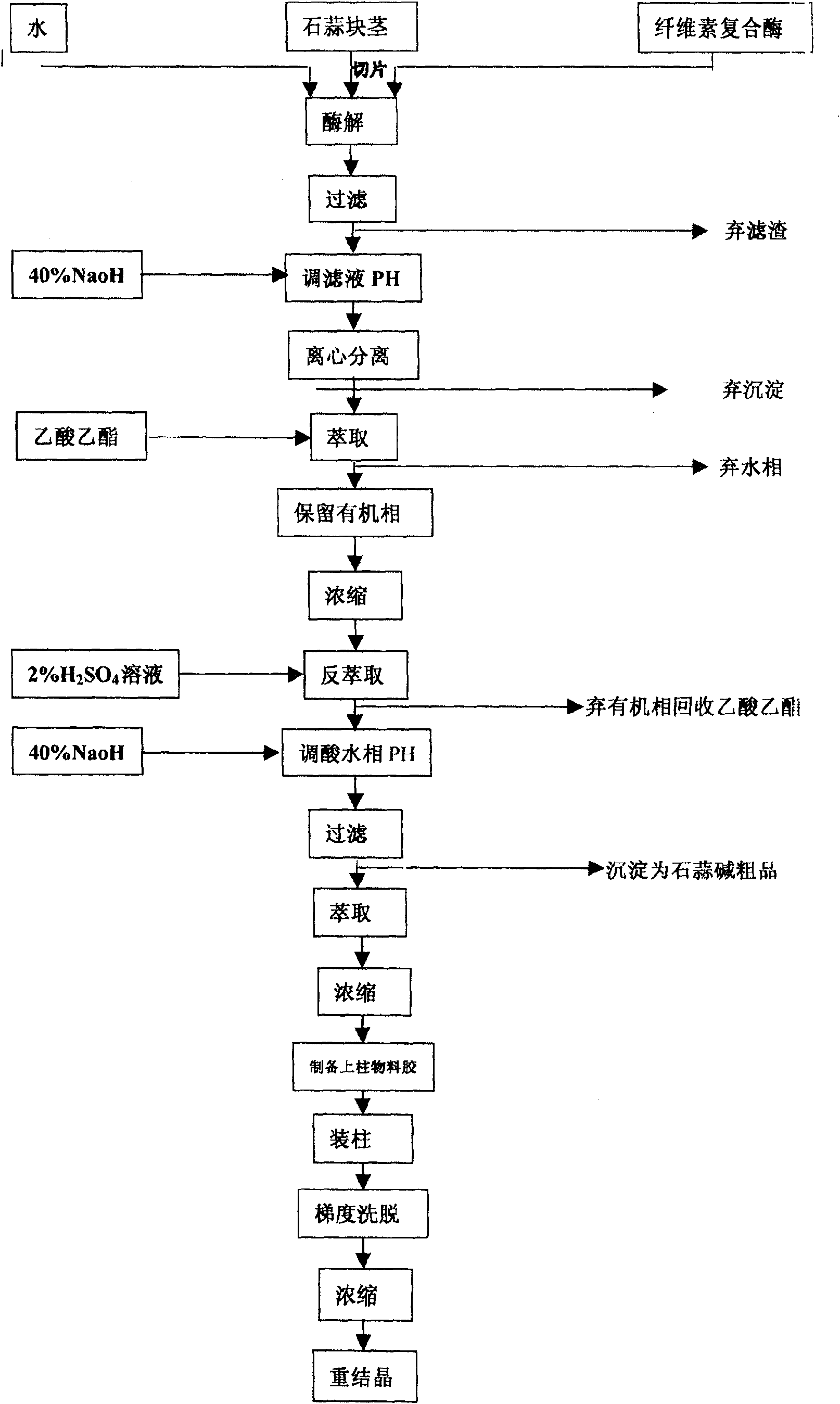
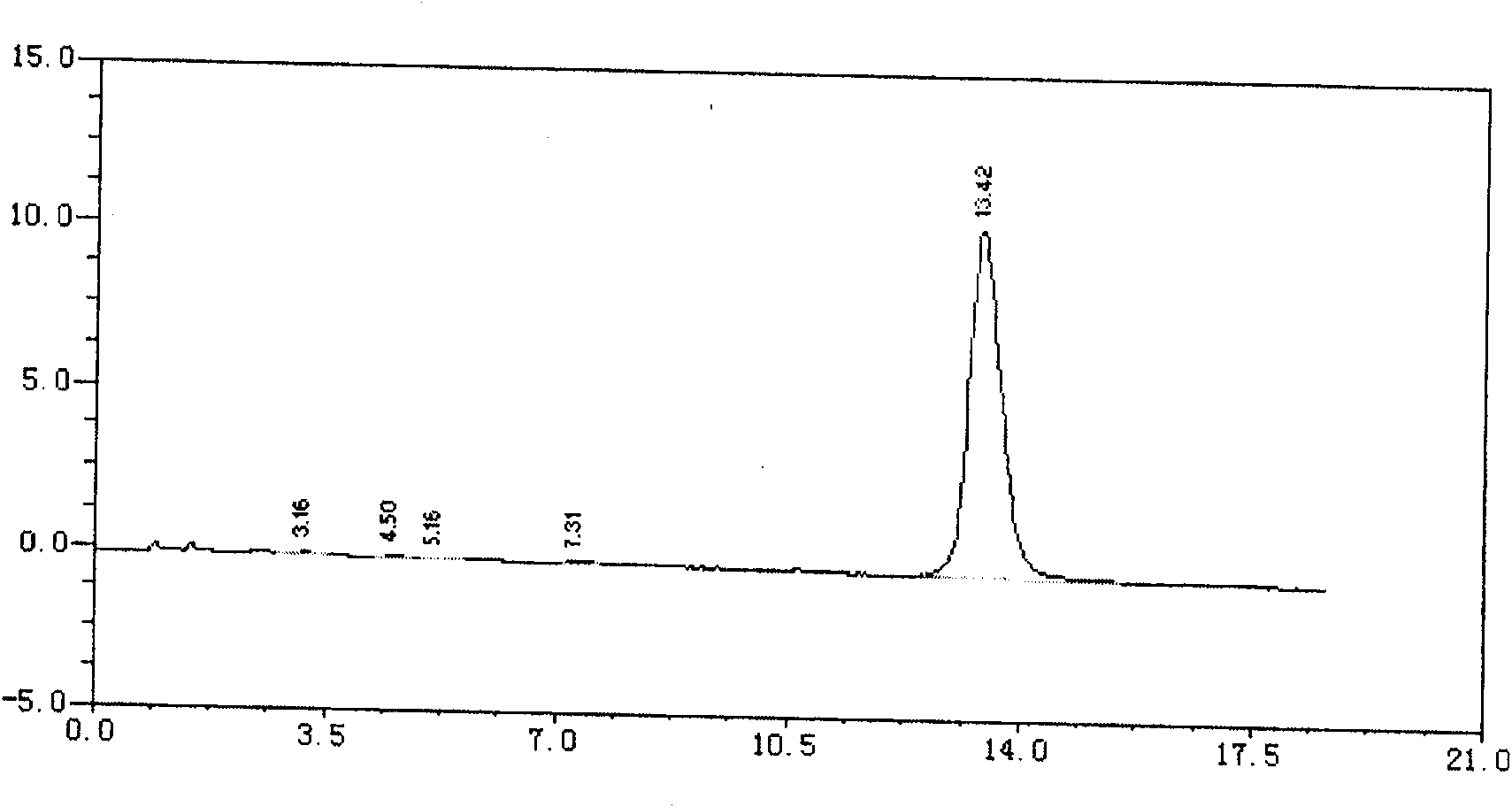
Technology for extracting dihydrogalanthamine from lycoris radiata genus plant
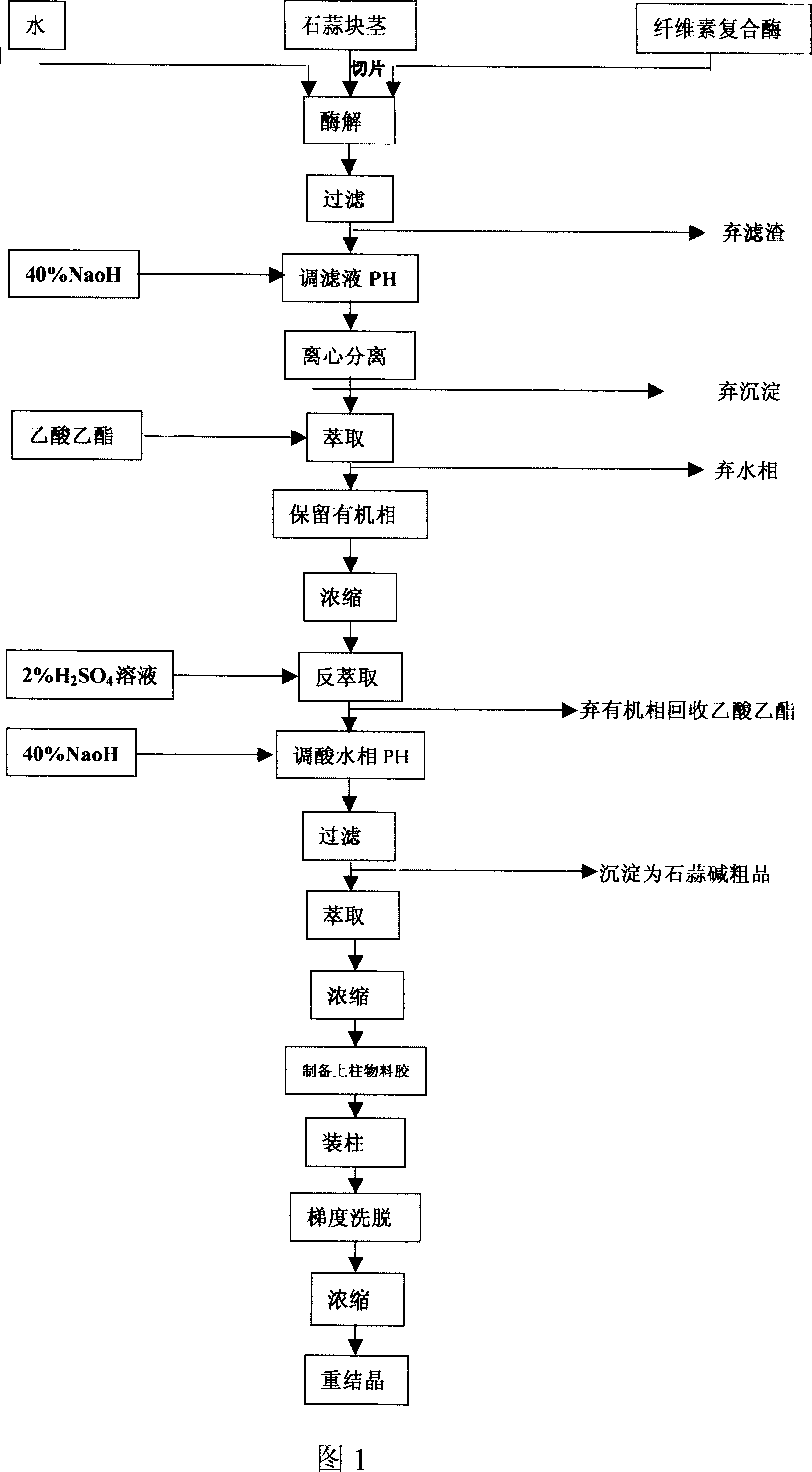
The invention discloses a process of extracting dihydrogalanthamine from Lycoris radiata plant, which in detail relates to a method for separating dihydrogalanthamine exquisite from alkaloid extracted from Lycoris radiata plant, and it is a recombined method of enzyme technology and chromatography. It comprises process and condition of extraction and separation of coarse lycorine, then extracting filtering liquid of coarse lycorine, condensing, preparing column material, loading column, eluting, condensing, recrystallizing and getting dihydrogalanthamine with purity of over 99%. The invention is different from method using organic solvent for dihydrogalanthamine extraction, and it can obtain coarse lycorine, coarse galantamin and dihydrogalanthamine with purity over 99%.
Owner:贵州芊芊园艺新技术发展公司
Galantamine formulations
Galantamine formulations substantially free of microcrystalline cellulose, lactose, and / or starch are described.
Owner:ACTAVIS GRP PTC EHF
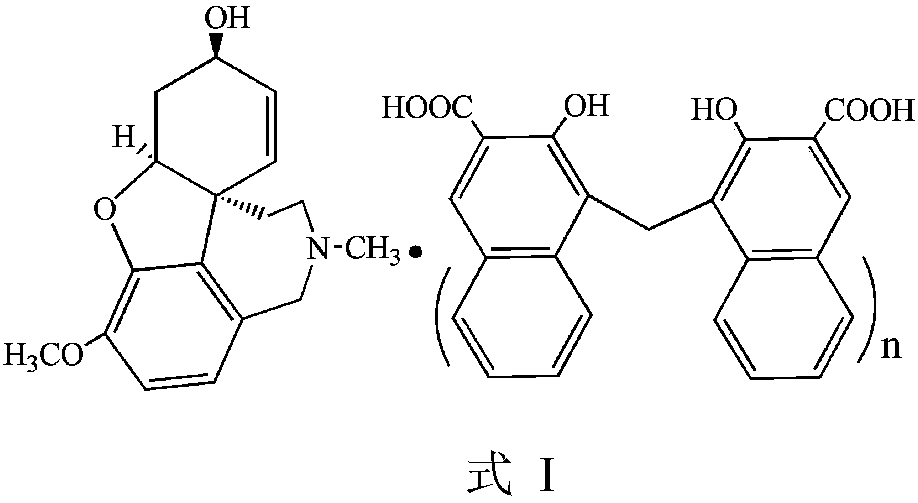
Galanthamine dihydroxy naphthoate and preparation method thereof
ActiveCN111233878AImprove stabilityReduce solubilityNervous disorderOrganic chemistry methodsEngineeringCombinatorial chemistry
The invention provides an amorphous galanthamine dihydroxy naphthoate compound and a preparation method thereof, the compound has low solubility, good stability and high safety, and a pharmaceutical preparation prepared by using the compound as an active component has good dissolution performance. In addition, the galanthamine dihydroxy naphthoate provided by the invention is simple in preparationprocess, high in product yield, high in purity and suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Galanthamine pamoic acid salt and preparation method thereof
ActiveCN111233877AImprove stabilityReduce solubilityNervous disorderOrganic chemistryEngineeringCombinatorial chemistry
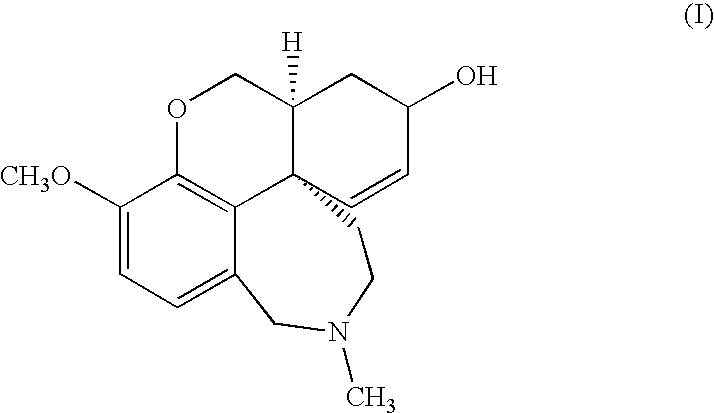
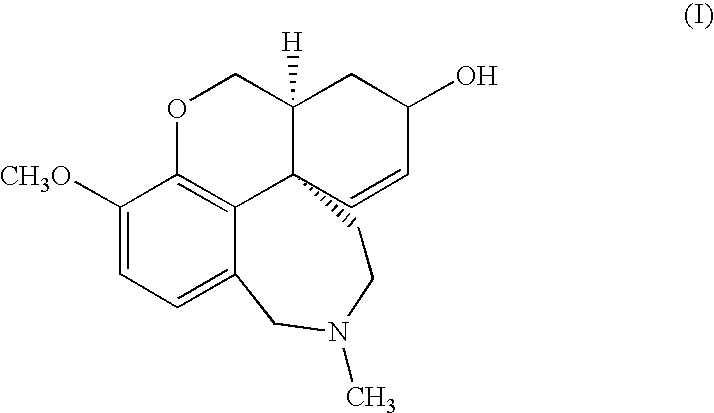
The invention provides a galanthamine pamoic acid salt and a preparation method thereof, the compound has a structure shown in formula I, and has good dissolution characteristic and good stability, and a tablet prepared by using the compound as an active ingredient has slow dissolution performance and embodies an excellent slow release effect. In addition, the galanthamine pamoic acid salt provided by the invention has the advantages of simple preparation process, high product yield and high purity, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Novel pharmaceutical compositions and methods for treating mental, behavioral, cognitive disorders
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMA TECH INC
Skin paste capable of improving dementia, delusion and myasthenia due to cholinergic
InactiveCN101455651AImprove the quality of lifeReduce the burden onNervous disorderSheet deliveryLife qualitySolvent
A skin patch which improves symptoms of ashronesia, delusion and myasthenia caused by cholinergic deficit is composed of an antiseizing layer, a medicine storing layer and a back lining layer, wherein the medicine storing layer comprises medicine, dissolvent and pressure-sensitive adhesive. The invention is characterized in that the medicine is galantamin or hydrobromate thereof. The galantamin or hydrobromate thereof is 0.007mg-10mg / cm<2>, and the medication area is 2.5-70cm<2>. The pressure-sensitive adhesive is selected from polyisobutene pressure-sensitive adhesive, emulsion and dissolvent type acrylate. When the pressure-sensitive adhesive is polyisobutene, the medicine storing layer is composed of medicine, dissolvent, pressure-sensitive adhesive, thickening agent, anti-oxidant and transdermal promoter. When the pressure-sensitive adhesive is emulsion type acrylate, the medicine storing layer is composed of medicine, dissolvent, pressure-sensitive adhesive, thickening agent and concentrated ammonia liquor. When the pressure-sensitive adhesive is dissolvent type acrylate, the medicine storing layer is composed of medicine, dissolvent, and pressure-sensitive adhesive. The skin patch of the invention can effectively treat the symptoms of ashronesia, delusion and myasthenia caused by cholinergic deficit, increase the life quality of patient and alleviate the burden of kin and society.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
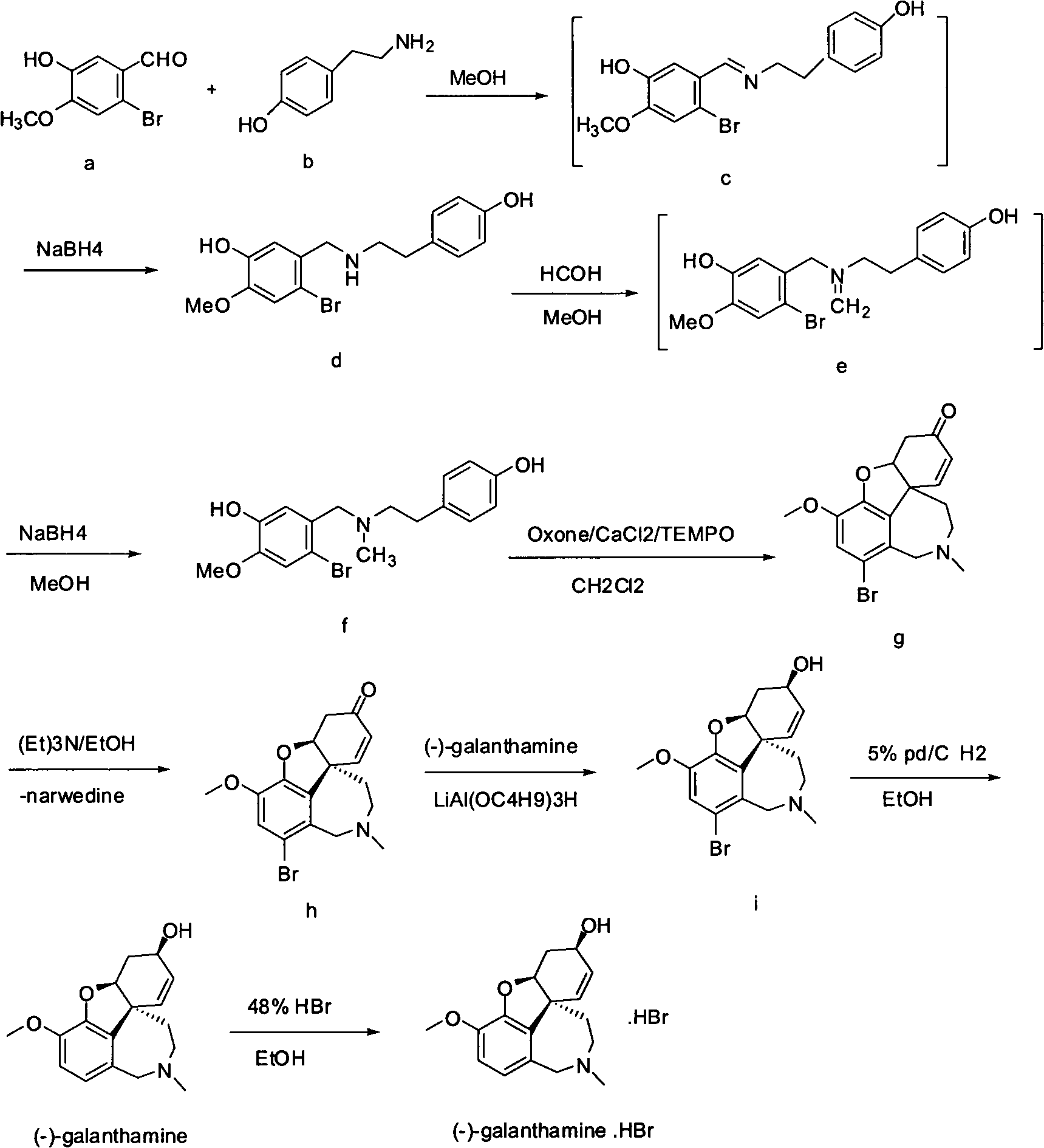
Synthesis method of galanthamine
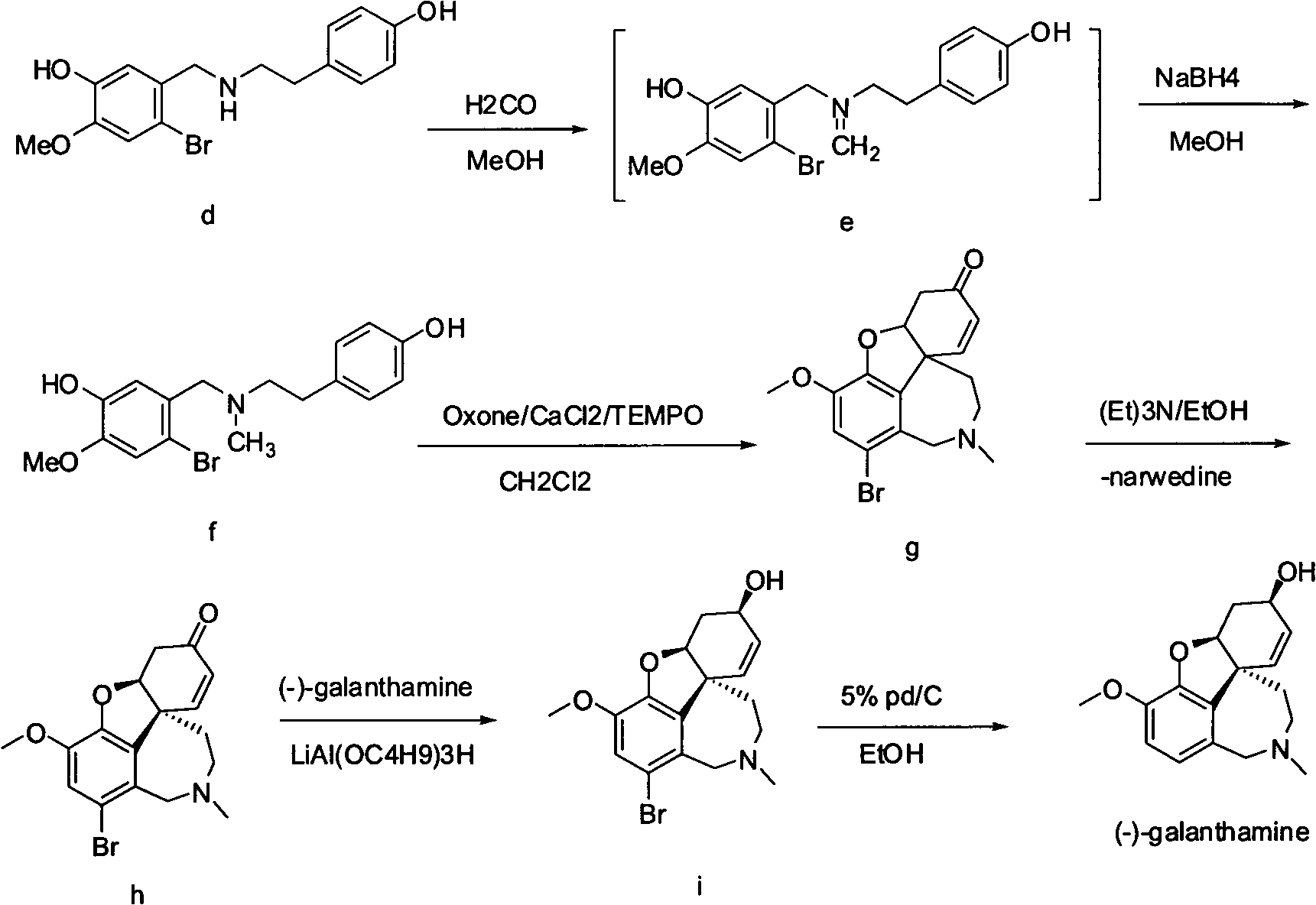
The invention provides a new method for the preparation of (-)-galanthamine. The method includes the following steps: preparing N-methyl-N-(4-hydroxyl phenethyl)-3-hydroxyl-4-methoxyl-6-bretylium (f) by condensation of N-(4-hydroxyl phenethyl)-3-hydroxyl-4-methoxyl-6-bretylium (d) as a starting material and formaldehyde, then preparing bromo-narwedine (g) by catalytic oxidation using an oxidizing agent (Oxone / CaCl2 / TEMPO) and a phase-transfer catalyst, transforming the bromo-narwedine to levo bromo-narwedine (h) by Shieh method, obtaining bromo-(-)-galantamine (i) by asymmetric reduction, and then obtaining the (-)-galanthamine by hydrogenated debromination.
Owner:TIANJIN HAIGELI TECH DEV
Method for comprehensive utilization of lycoris resource
The invention provides a method for preparing galanthamine and a water retaining gel from lycoris. The method comprises the following steps: refluxing dried lycoris by using a 70-90% alcoholic solution to extract lycorine, performing column separation to obtain galanthamine, washing lycoris residues by using an acrylic acid solution, and compounding with a catalyst to obtain the water retaining gel. According to the method, the starch in the lycoris is fully used for preparing water retaining gel, and the pollution of lycoris wastes to the environment is avoided. An acrylic acid aqueous solution is directly used for washing to dissolve out starch, so that operation steps are reduced, the time for treating the lycoris wastes is shortened, and the production cost of lycorine is reduced.
Owner:徐州天骋智能科技有限公司
Galanthamine pamoate sustained-release microspheres for injection and preparation method thereof
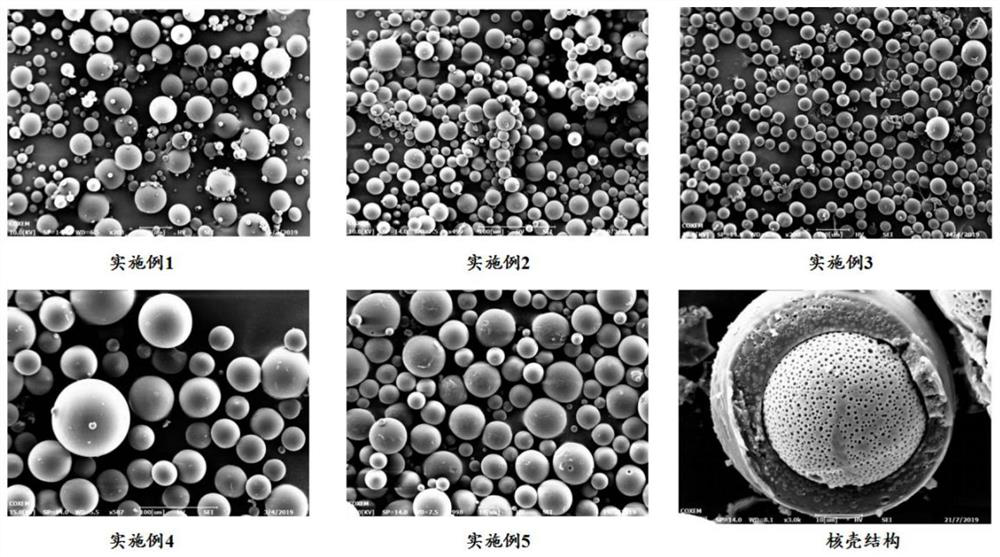
ActiveCN112823792AHigh drug loadingHigh encapsulation efficiencyNervous disorderPharmaceutical non-active ingredientsSolvent evaporationMicrosphere
The invention belongs to the technical field of drug sustained-release preparations, and particularly discloses galanthamine pamoate sustained-release microspheres for injection and a prpearation method thereof. The microspheres are composed of galanthamine pamoate and a lactide-glycolide copolymer. The galanthamine pamoate sustained-release microspheres for injection are prepared by adopting a mixed solvent system intervened O / W single emulsification-solvent evaporation method, and the microspheres are good in form, uniform in particle size, unique in core-shell structure, high in drug loading capacity and encapsulation efficiency and controllable in release period, and can meet the clinical requirements of different administration periods; and the preparation method of the microspheres is simple and feasible, and meets the requirements of modern industrial large-scale production.
Owner:LUNAN PHARMA GROUP CORPORATION
Skin Lightening Compositions with Acetylcholinesterase Inhibitors
ActiveUS20100143277A1Reduce the impactReduce melanin productionCosmetic preparationsBiocideCholinesterase inhibitionDepressant
Skin lightening additives and skin lightening compositions having an acetylcholinesterase inhibitor are described. The compositions are suitable for topical application and may comprise inhibitors like galanthamine, taspine or both.
Owner:CONOPCO INC D B A UNILEVER
Process For The Preparation Of Galanthamine Hydrobromide
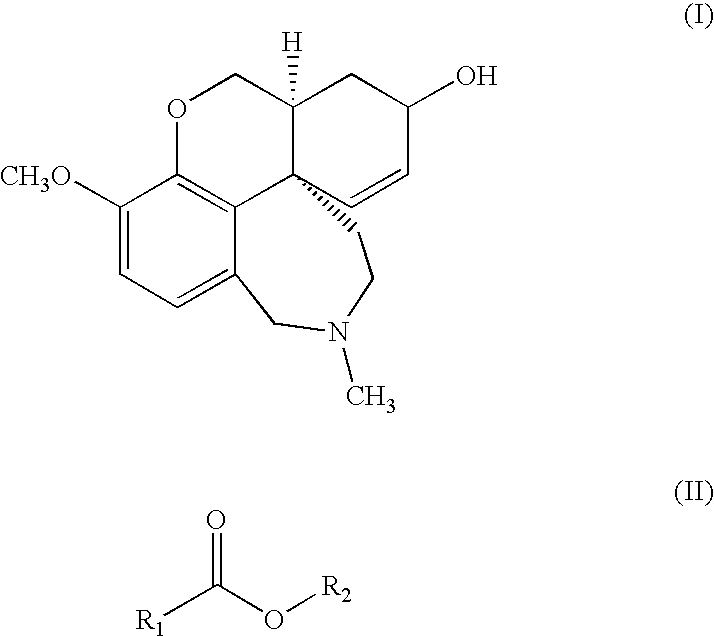
A process for the purification of galanthamine (I) comprising precipitation of galanthamine hydrobromide from a mixture of alkaloids obtained from a plant of the Amaryllidaceae family containing galanthamine, treatment of the hydrobromide with alkali, extraction and crystallization of galanthamine with a solvent of general formula (II), in which R1 is hydrogen or methyl and R2 is selected from n-butyl, isobutyl, sec-butyl and t-butyl. The resulting pure galanthamine can be conveniently used for the preparation of galanthamine hydrobromide.
Owner:INDENA SPA
Cholinesterase inhibitors in liposomes and their production and use
Owner:SANOCHEMIA PHARMA AG
Enhanced brain bioavailability of galantamine by selected formulations and transmucosal administration of lipophilic prodrugs
ActiveUS11077119B2Increase concentrationImprove solubilityPowder deliveryNervous disorderLipophilic prodrugsBioavailability
A method of treating a subject for a brain disease associated with cognitive impairment, including administering to a subject a chemical substance according to GLN-1062 or salt thereof:wherein the treatment includes transmucosal administration of a therapeutically effective amount of GLN 1062 or salt thereof in the oral cavity of the subject.
Owner:NEURODYN LIFE SCI
Galantamin hydrobromide production method
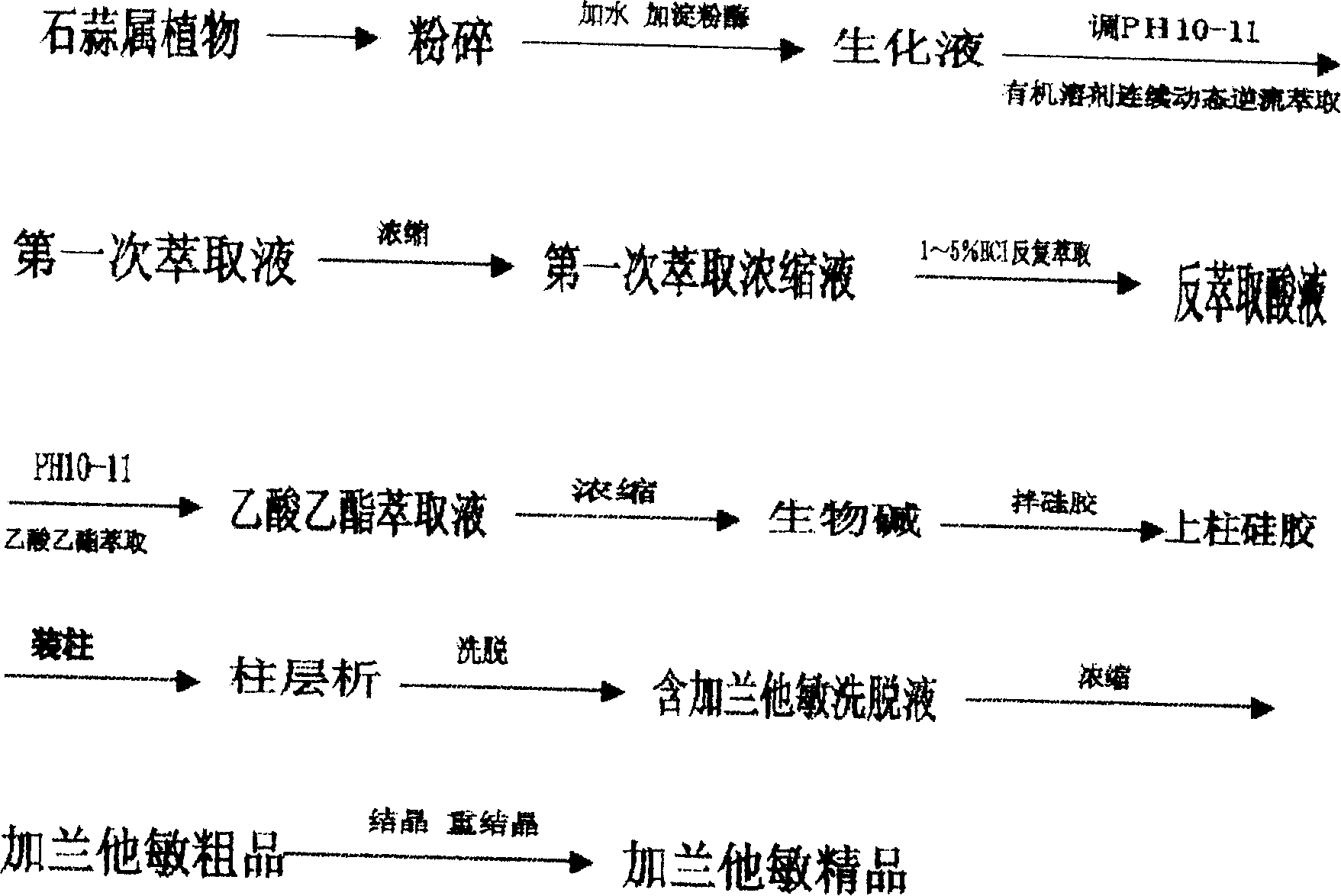
The invention discloses a galantamin hydrobromide producing method for treating senile dementia, which comprises the following steps: adopting the method of enzyme reaction, continuous kinesis flow upstream extracting and column chromatography to extract galantamin with purity of 99% from Amaryllidaceae plant; Using galantamin with purity of 99 percent as raw material; obtaining galantamin hydrobromide with purity of 98.5. percent. The invention discloses an extracting galantamin of purity at 99% process flow and technological condition from Amaryllidaceae plant, which adapts for industrialization production.
Owner:贵州芊芊园艺新技术发展公司
Novel method for preparing galanthamine sustained-release microspheres
ActiveCN111643483AResidue reductionHigh encapsulation efficiencyNervous disorderPharmaceutical non-active ingredientsMicrospherePropylene carbonate
The invention discloses a novel method for preparing galanthamine sustained-release microspheres, and particularly relates to a method for preparing galanthamine microspheres by adopting a novel solvent propylene carbonate. Galanthamine pamoate is used as a raw material, and the galanthamine sustained-release microspheres prepared by using an emulsification-solvent volatilization method have the advantages of high encapsulation efficiency, high product yield, less residual solvent and the like.
Owner:UNIV OF JINAN
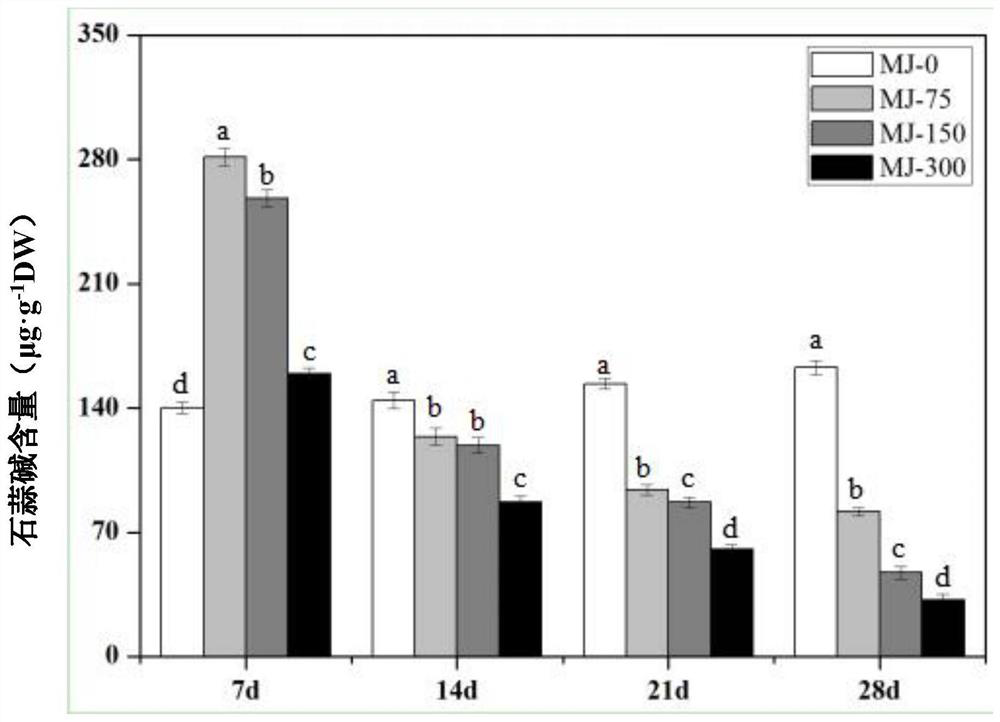
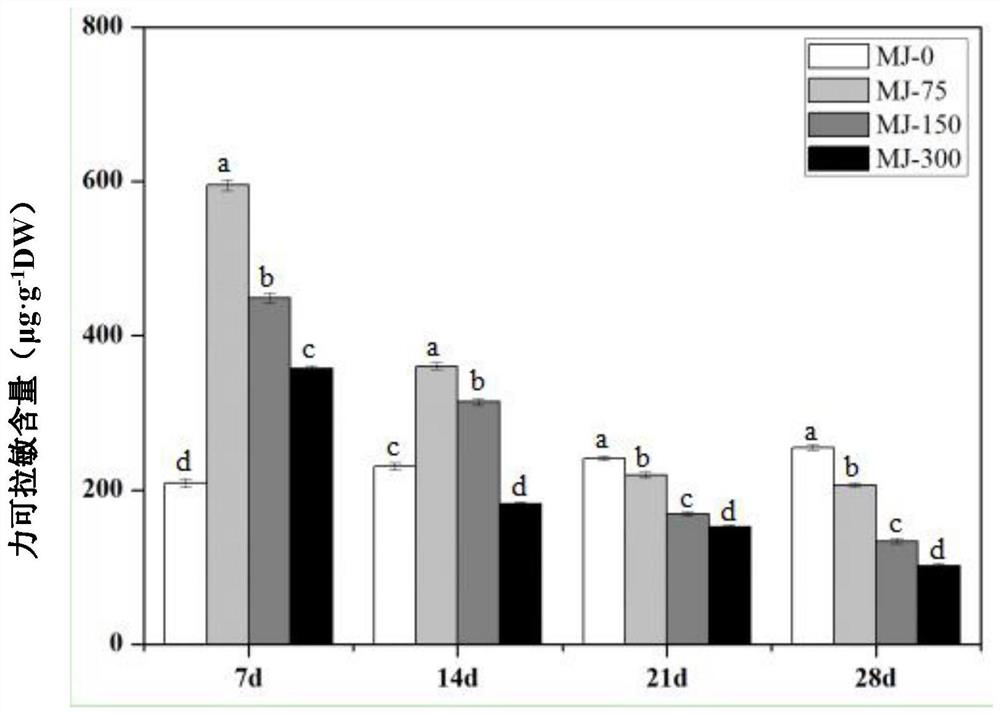
Method for increasing content of alkaloid in lycoris radiata through exogenous substance
InactiveCN112753410AIncrease alkaloid contentShorten the timePlant tissue cultureHorticulture methodsMetaboliteLycoris radiata
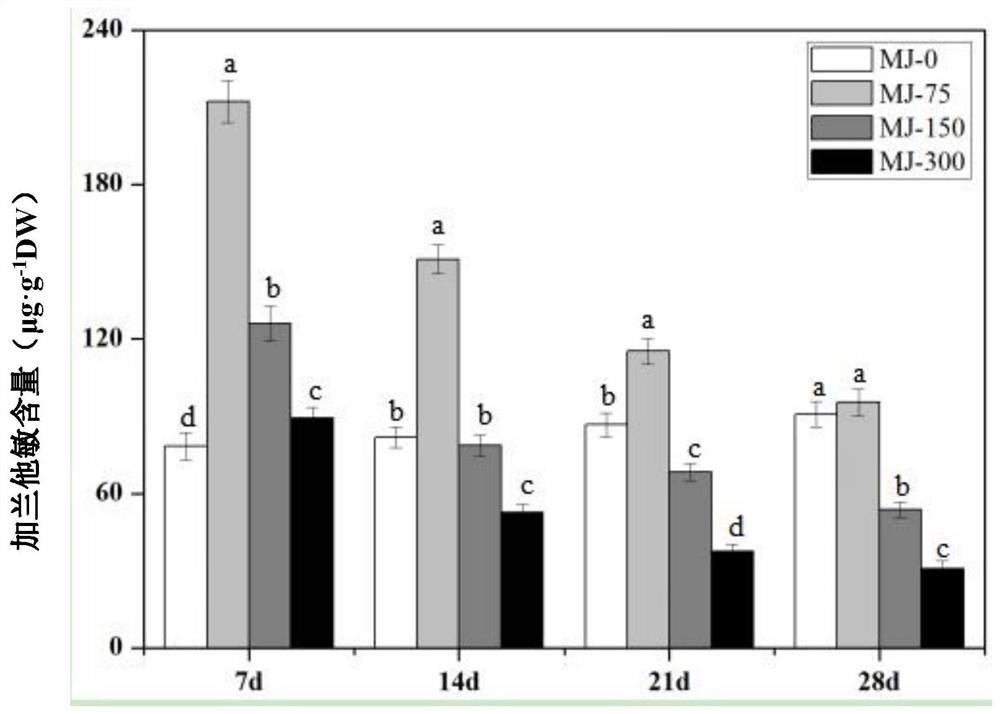
The invention provides a method for increasing the content of alkaloid in lycoris radiata through an exogenous substance, and relates to the field of plant growth and secondary metabolite accumulation regulation. According to the method for increasing the content of the alkaloid in the lycoris radiata through the exogenous substance, 3-month-to-one-year-old tissue culture seedlings of the lycoris radiata are treated through the exogenous substance 4 '-O-methyl norlinaldine and / or jasmonic acid methyl ester, so that the content of three alkaloids, that is, galanthamine, lycorine and lycorine which have important medicinal values in the lycoris radiata are effectively increased.
Owner:SHANGHAI ELITE AGRI SCI TECH GROUP +1
Method for separating high-purity galanthamine from short-tube lycoris crude extract
A process for separating high-purity galantamin from the coarse extract of short-tube lycoris by counter-current chromatography includes such steps as preparing two-phase solvent system from 2,3,044 of paraffin, halohydrocarbon, emtrol, lipoketone, lipoester, ether, buffering liquid of inorganic salt and water, waving, laying aside for layering, dissolving the coarse extract of short-tube lycoris, and separating. Its advantages are high separating capacity and recovery rate, and less solvent consumption.
Owner:浙江一新制药股份有限公司
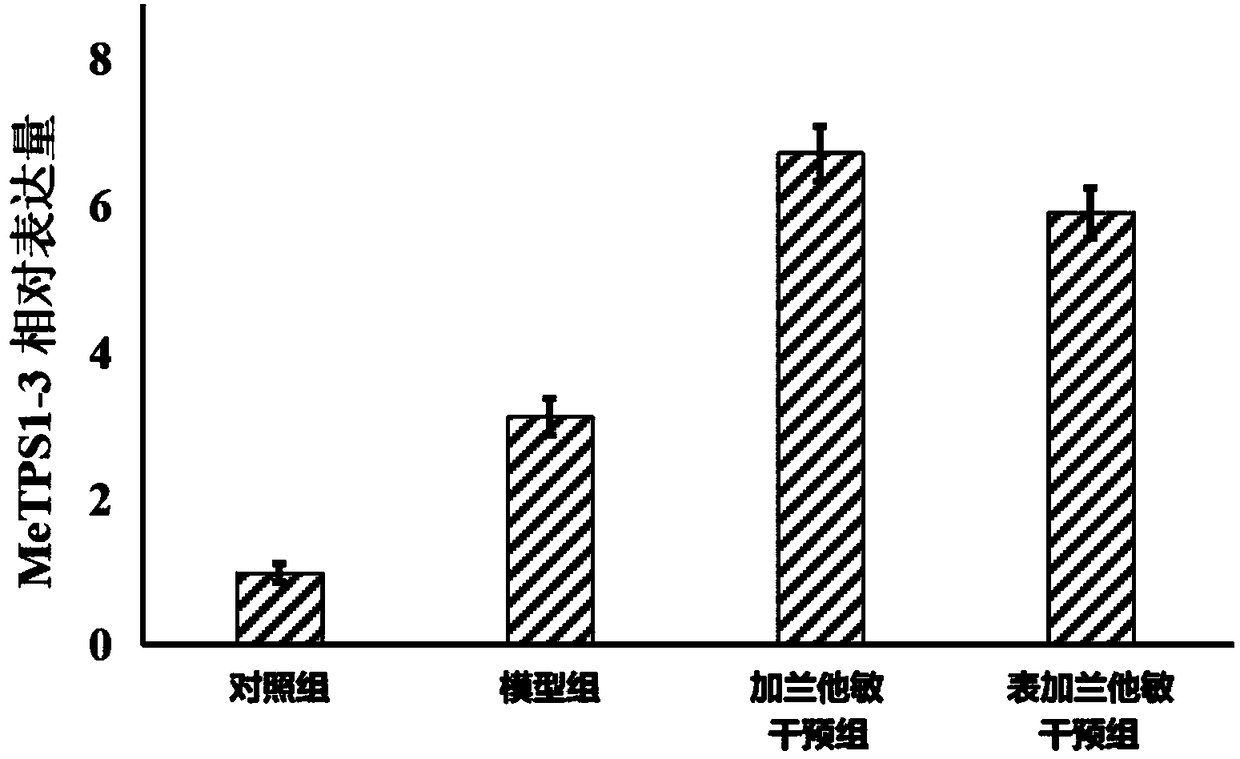
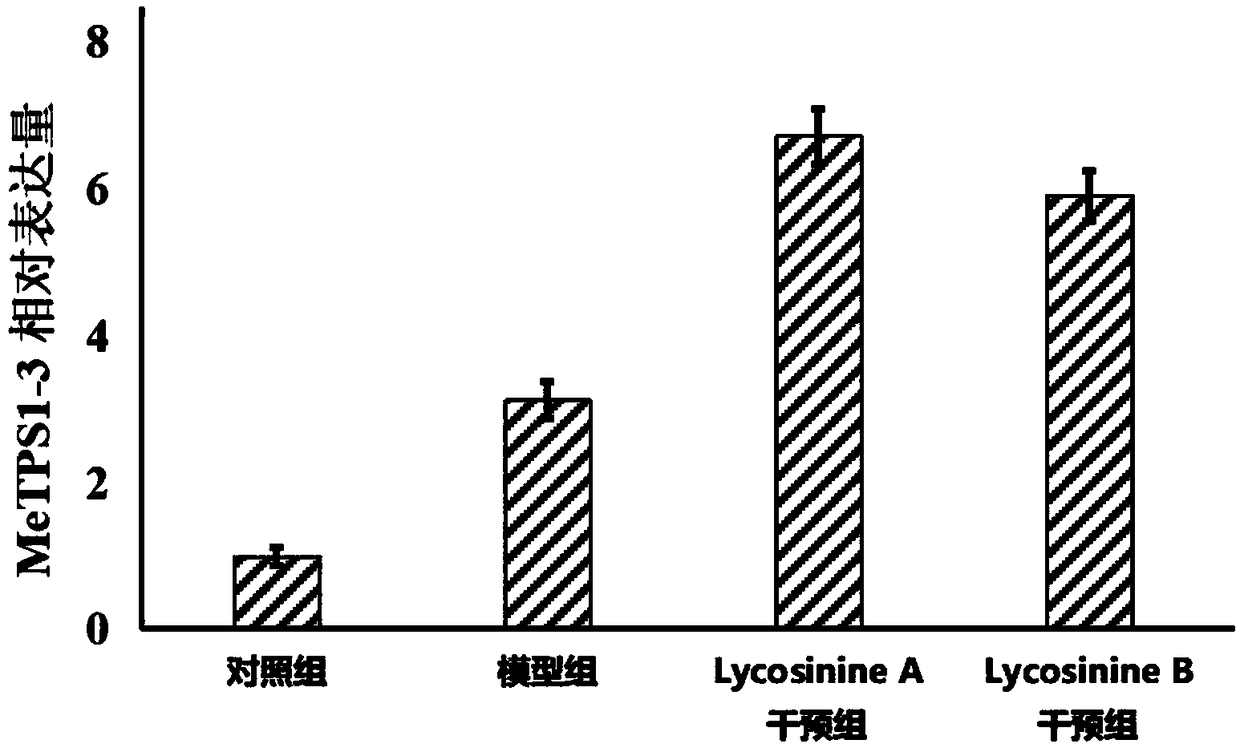
Application of manihot esculenta crantz MeTPS1-3 gene expression activator in preparing fertilizer for enhancing drought resistance of manihot esculenta crantz
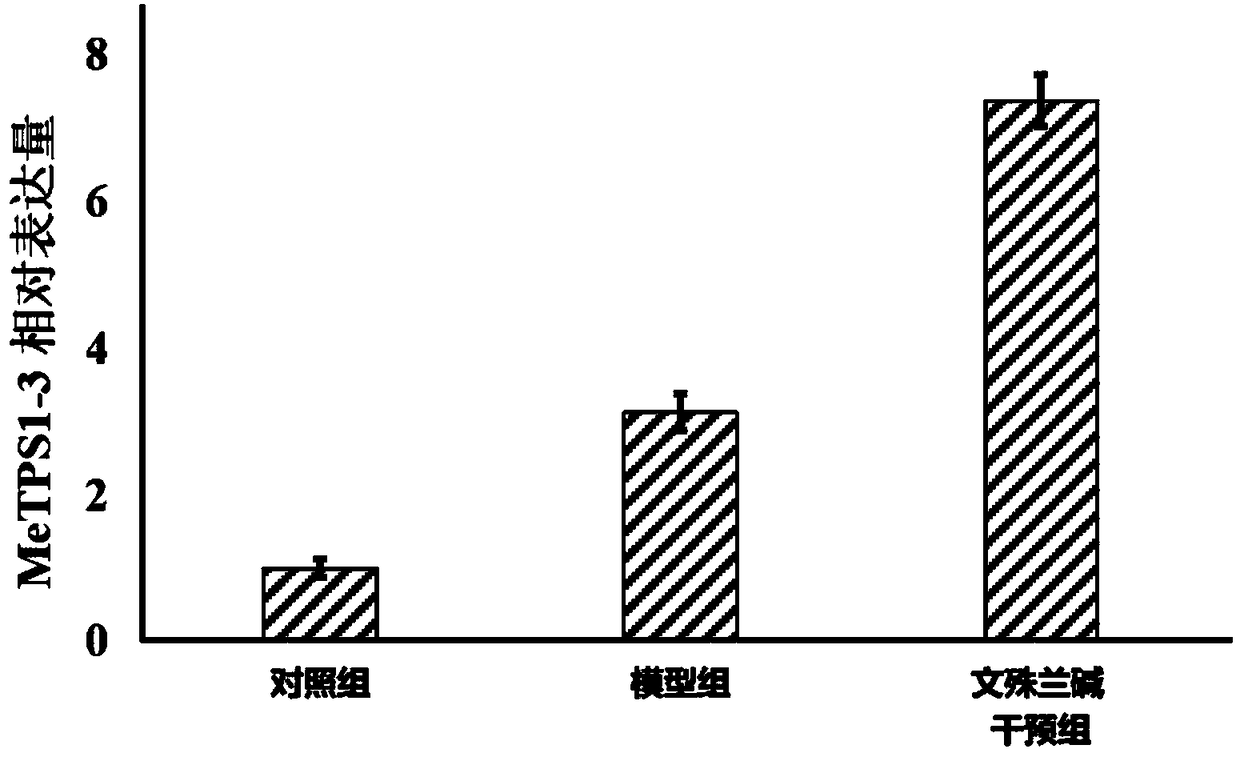
InactiveCN108178696AImprove drought resistanceImprove the immunityPlant growth regulatorsBiocideFertilizerDrought resistance
The invention discloses application of a manihot esculenta crantz MeTPS1-3 gene expression activator in preparing fertilizer for enhancing the drought resistance of manihot esculenta crantz. As known,expression of MeTPS1-3 can be irritably added by the manihot esculenta crantz under a drought situation, so that resistance to drought stress can be increased. The invention discovers that after intervention of galantamine and epi-galantamine is given, the expression of the MeTPS1-3 is remarkably and further increased, and meanwhile, the resistance to the drought stress is remarkably enhanced, sothat the galantamine and the epi-galantamine can be used for preparing the fertilizer capable of increasing the drought resistance of the manihot esculenta crantz. Fertilizer additives or fertilizerpreparation ingredients can also be added in the fertilizer, the fertilizer additives can be human and animal excreta, and the fertilizer preparation ingredients can be pulverized fuel ash, plant ash,bentonite, kaolin, attapulgite clay or plant straw.
Owner:李冬
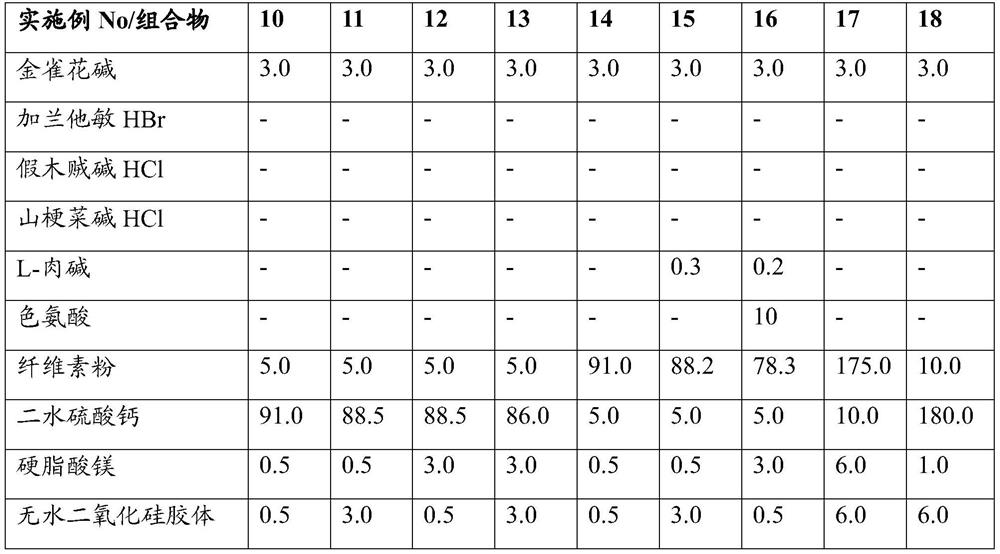
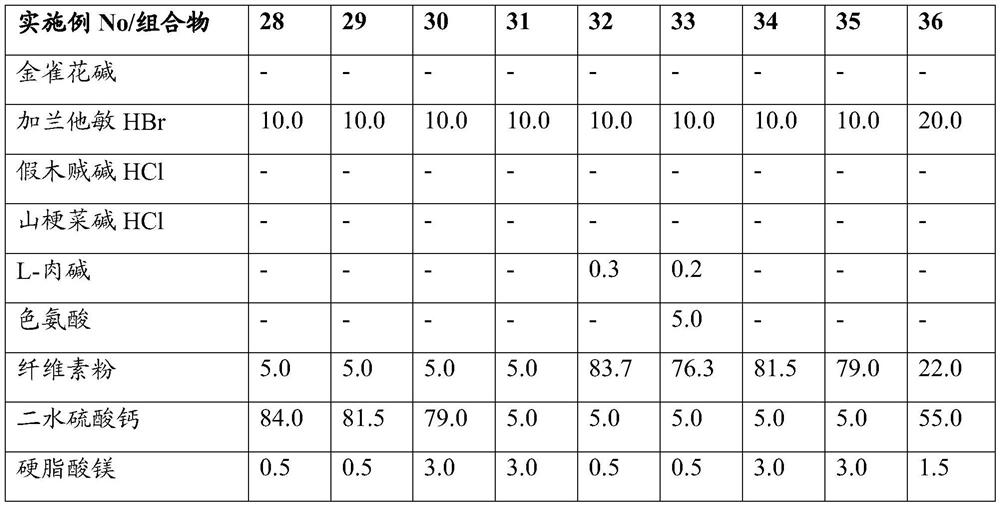
Oral pharmaceutical composition with plant alkaloid for treatment of dependencies
PendingCN113194930AReduced responseGood disintegrationNervous disorderPill deliveryAnabaseineTryptophan
This invention is related to an oral pharmaceutical composition that contains a cholinergic agent, a natural plant alkaloid in particular, selected from the group of lobeline, anabasine, cytisine, galantamine or their acceptable salts, in the form of tablets and capsules. The excipients of the developed oral composition include cellulose powder, calcium sulphate, silica colloidal and magnesium stearate, the total content of cellulose powder and calcium sulphate dihydrate being from 64,5 to 97,5 % of the mass of the dosage form and at least 90% of the alkaloid particles being < 100um. The oral composition contains also at least one biologically active amino acid selected from: L-carnitine, tryptophan or a combination of them. The oral composition according to this invention achieves uniform distribution of the active substance in the composition, as well as stability of the composition due to the included excipients selected so as to react to a minimum with the alkaloid to form the qualitative and quantitative related substances admissible for the pharmaceutical composition. The composition according to this invention is applicable in the treatment of dependency and addiction to nicotine, tobacco products and alcohol.
Owner:索非药剂有限公司
Isolation of Galanthamine From Biological Material
The subject matter of present invention relates to the process for isolation and purification of galanthamine and its derivatives produced by numerous plants.
Owner:IVAX PHARMA
Purification production process of galanthamine
InactiveCN113603699AEasy to separate and extractEasy to operateOrganic chemistry methodsBiotechnologyAlcohol
The invention relates to a purification production process for galanthamine. According to the purification production process provided by the invention, galanthamine is purified from lycoris radiata plants; crude extraction and fine extraction are mainly included in the process; used main raw materials comprise lycoris radiata, methanol, chloroform, dichloromethane, chromatographic silica gel, absolute ethyl alcohol and hydrobromic acid; only equipment such as an extraction kettle and a concentration kettle is needed; and thus, the raw materials are convenient to obtain, equipment investment is low, operation is convenient, separation and extraction effect on galanthamine is good, the purity of an extracted product is high, production cost is low, and the process is suitable for industrial and large-scale production.
Owner:怀化市盛德生物科技有限责任公司
A method for preparing galantamine sustained-release microspheres
ActiveCN111643483BHigh encapsulation efficiencyHigh yieldNervous disorderPharmaceutical non-active ingredientsMicrosphereProcess engineering
The invention discloses a new method for preparing galantamine sustained-release microspheres. Specifically, a new solvent propylene carbonate is used to prepare galantamine microspheres. Using galantamine pamoate as raw material, the galantamine sustained-release microspheres prepared by emulsification-solvent evaporation method have the advantages of high encapsulation efficiency, high product yield, and less residual solvent.
Owner:UNIV OF JINAN
A method for detecting galantamine in sudden laughter
ActiveCN108717002BShorten the timeHigh sensitivityPreparing sample for investigationMaterial electrochemical variablesScreen printingEngineering
The invention discloses a method for detecting galantamine in lycoris aurea, and relates to plant sample handling, preparation of a nanogold printing electrode sensor and electrochemical detection. According to the method, the nanogold-modified printing electrode sensor is adopted to fully absorb in a sample extracting solution, then the nanogold printing electrode sensor is subjected to differential pulse voltammetry scanning to measure current values at oxidation peaks, within a detectable range, the content of the galantamine in the lycoris aurea absorbed by the nanogold printing electrodesensor is in a linear relation with the current intensities at the oxidation peaks, and according to a working curve, the content of the galantamine in the detected lycoris aurea can be calculated. The method has the advantages that the nanotechnology and the silk-screen printing technology are combined by the prepared nanogold printing electrode sensor, sensitivity, in galantamine concentration detection, of the sensor can be improved remarkably, and the minimum limit of detection of the sensor can reach to 0.013 microns.
Owner:上海联星医药科技有限公司
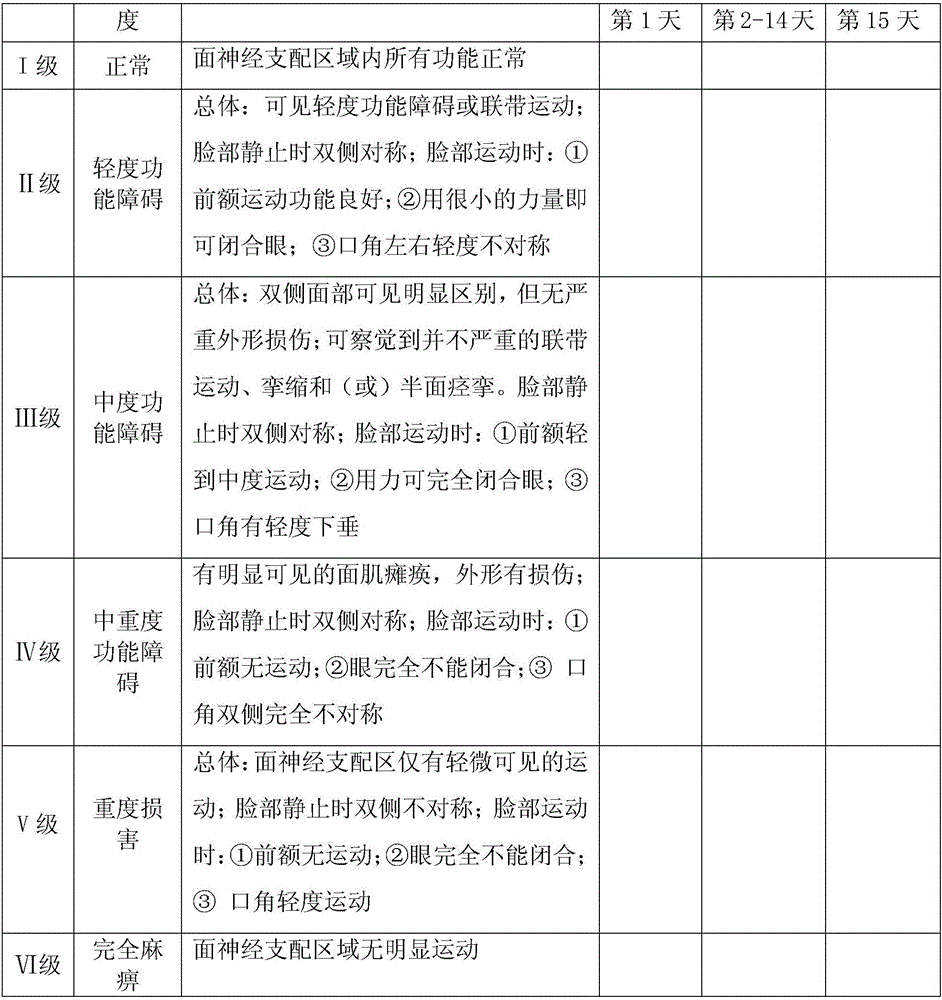
Ointment used for treating facial paralysis and preparation method thereof
ActiveCN106265678AReasonable compositionEasy to useNervous disorderHydroxy compound active ingredientsSide effectCure rate
The invention provides an ointment capable of effectively treating facial paralysis and a preparation method thereof. The ointment comprises the following components: hexadecanol, glycerin monostearate, octadecanoic acid, methyl silicone oil, liquid paraffin, glycerol, ethylhexyl p-hydroxybenzoate, Tween 80, galanthamine injection and laurocapram. After being prepared, the ointment is applied to the face and comes into full contact with and is fully absorbed by acupoints and local neuromuscular junction parts on the face through massaging, so that the aims of interacting and complementing each other can be achieved, thus giving play to the two advantages of strengthening the body resistance and eliminating evil. The ointment has the characteristics of good curative effects, high cure rate, safety in use and no toxic or side effect.
Owner:陕西省中医医院
Technology for extracting dihydrogalanthamine from lycoris radiata genus plant
The invention discloses a process of extracting dihydrogalanthamine from Lycoris radiata plant, which in detail relates to a method for separating dihydrogalanthamine exquisite from alkaloid extractedfrom Lycoris radiata plant, and it is a recombined method of enzyme technology and chromatography. It comprises process and condition of extraction and separation of coarse lycorine, then extractingfiltering liquid of coarse lycorine, condensing, preparing column material, loading column, eluting, condensing, recrystallizing and getting dihydrogalanthamine with purity of over 99%. The inventionis different from method using organic solvent for dihydrogalanthamine extraction, and it can obtain coarse lycorine, coarse galantamin and dihydrogalanthamine with purity over 99%.
Owner:贵州芊芊园艺新技术发展公司
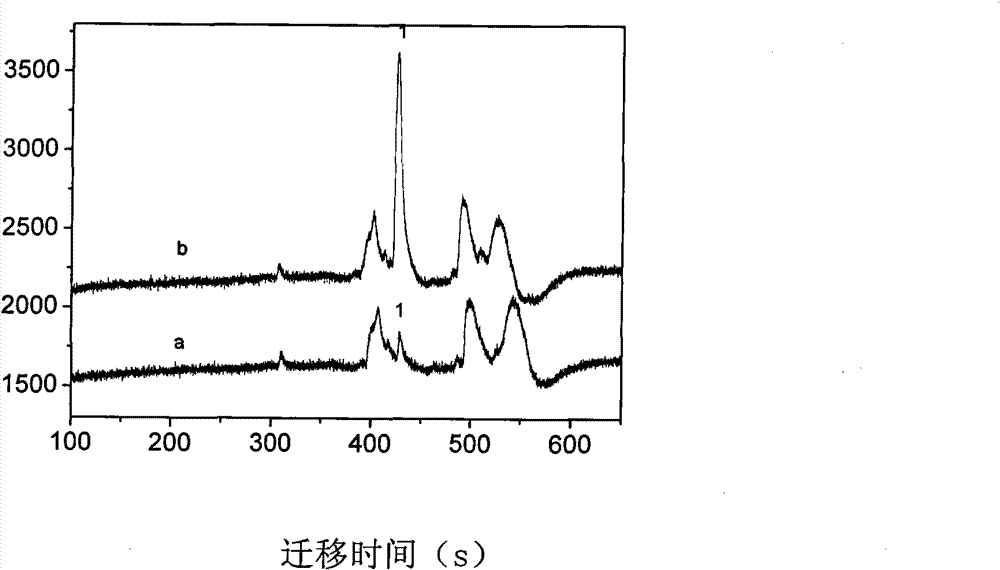
Capillary tube electrophoresis electrochemiluminescence detecting method for galantamine hydrobromide in traditional Chinese medicinal short-tube lycoris extract
InactiveCN102335297BHigh selectivityImprove anti-interference abilityNervous disorderChemiluminescene/bioluminescenceBiotechnologyElectrophoreses
The invention provides a capillary tube electrophoresis electrochemiluminescence detecting method for galantamine hydrobromide in a traditional Chinese medicinal short-tube lycoris extract. In the method, the detection limit of the galantamine hydrobromide is 1.0*10<-9> mol / L, being 1-2 orders of magnitude lower than that of a common mass spectrometric detection method; the linear range is 2.5*10<-7>-5.0*10<-5> mol / L, overcrossing two orders of magnitude; the sensitivity of the galantamine hydrobromide is detected by using pyridine-ruthenium electrochemiluminescence; efficient separation of the galantamine hydrobromide is realized with a phosphate running buffer solution; the sample size is upgraded by using sodium, and derivation of a sample to be detected is not required; and the traditional Chinese medicinal short-tube lycoris extract solution is directly used for detecting after being filtered and diluted without complex procedures such as drying, purifying and the like. In the invention, a capillary tube electrophoresis electrochemiluminescence detecting technology is applied to the detection of the galantamine hydrobromide in the traditional Chinese medicinal short-tube lycoris extract for the first time.
Owner:CHANGCHUN INST OF TECH +1
A kind of extraction method of Lycoris plant protoplast
The invention provides a method for extracting protoplasts of Lycoris plants, the method comprising the following steps: (1) treating Lycoris plant seedlings in the dark for 10-12 hours, taking young leaves or bulbs and chopping them; (2) using a reagent A process the fragment material obtained in step (1), and slowly shake it on a shaking table to release protoplasts; (3) filter the protoplast solution obtained in step (2) with a 40-50 μm filter screen, and centrifuge the filtrate for 8-10min , wash the precipitate with Reagent B, centrifuge repeatedly, and finally use Reagent C to suspend the protoplasts. The extraction method of Lycoris plant protoplasts of the present invention has a large number of protoplasts and good integrity, can be directly used for the detection of Lycoris alkaloids galantamine, and can be used for protoplast fusion and protoplast transformation Provide important materials.
Owner:SHANGHAI ACAD OF AGRI SCI
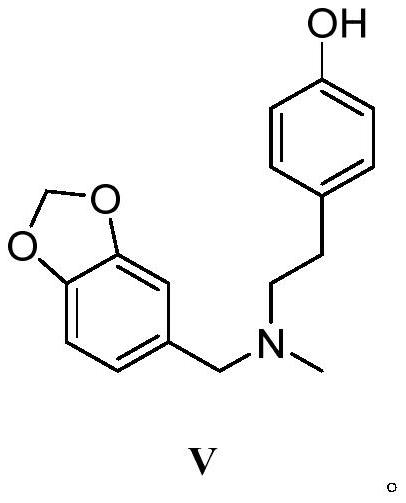
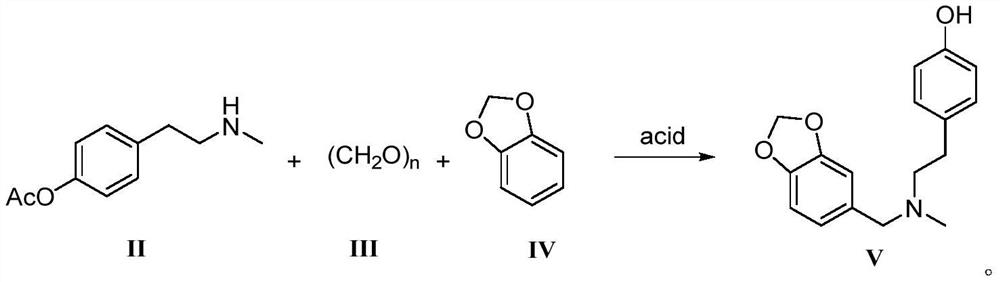
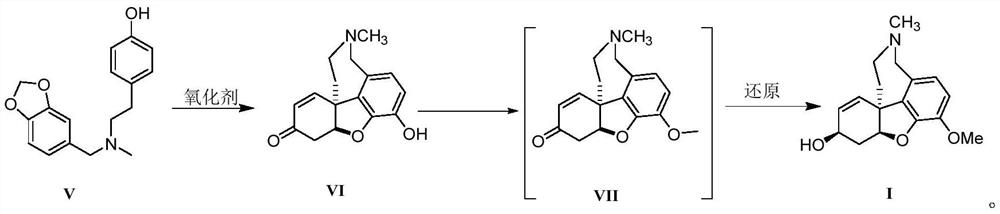
Galanthamine intermediate compound V
PendingCN112521364ASimple and fast operationSimple and efficient operationOrganic chemistry methodsBulk chemical productionPolyoxymethylenePtru catalyst
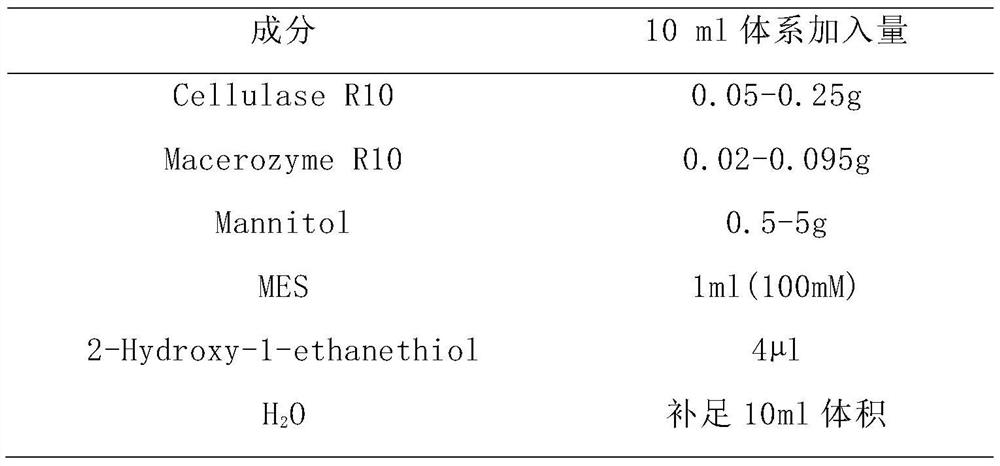
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a galanthamine intermediate compound V. According to the invention, 4-(2-(methylamino)ethyl)phenyl acetate,paraformaldehyde and 1,3-benzodioxole are used as raw materials to synthesize the novel intermediate compound V. The invention also provides a novel method for synthesizing galanthamine by using the intermediate. According to the method, use of dangerous chemical reagents is avoided, the synthesized intermediate does not generate new impurities, a green catalyst is used for replacing a traditionalcatalyst, a reaction is milder, economical and environment-friendly, yield is higher, and the method is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com