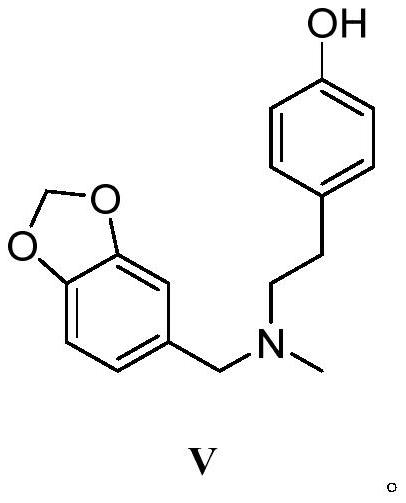
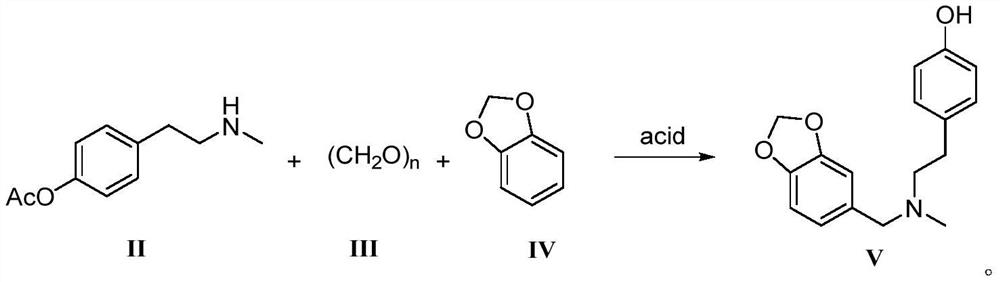
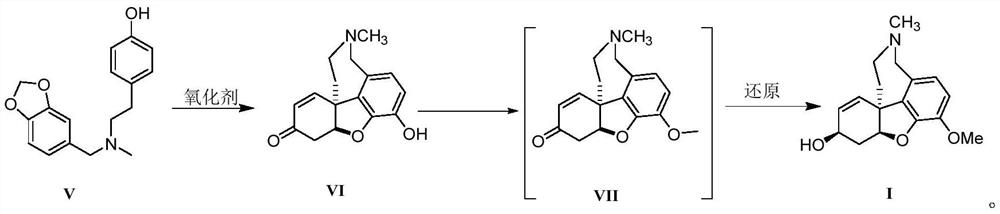
Galanthamine intermediate compound V
A technology of galantamine and compound, applied in the field of galantamine intermediate compound V, can solve the problems of serious environmental pollution, high technical requirements and high production cost, achieve economical and environmental protection yield, high product purity and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add compound II (19.3g, 0.10mol), paraformaldehyde (13.51g, 0.15mol), compound IV (14.64g, 0.12mol), p-toluenesulfonic acid (3.44g, 0.02mol), 100mL toluene into the there-necked flask . Stir to dissolve, heat to reflux, remove the generated water from the reaction system with a water separator, stop heating when the water in the water separator no longer increases, and slowly cool down to room temperature. Add 100 mL of ethyl acetate, shake vigorously and let it stand still, collect the organic phase and dry it with anhydrous sodium sulfate. The desiccant was removed by filtration and concentrated under reduced pressure to obtain a white solid powder with a yield of 92.5% and an HPLC purity of 99.92%.
Embodiment 2
[0059] Add compound II (19.3 g, 0.10 mol), paraformaldehyde (9.91 g, 0.11 mol), compound IV (14.64 g, 0.12 mol), benzenesulfonic acid (3.16 g, 0.02 mol), and 100 mL of toluene into the three-neck flask. Stir to dissolve, heat to reflux, remove the generated water from the reaction system with a water separator, stop heating when the water in the water separator no longer increases, and slowly cool down to room temperature. Add 100 mL of dichloromethane, shake vigorously and let stand, collect the organic phase, dry with anhydrous sodium sulfate, filter to remove the desiccant, and concentrate under reduced pressure to obtain a white solid powder with a yield of 85.1% and an HPLC purity of 99.87%.
Embodiment 3
[0061] Add compound II (19.3 g, 0.10 mol), paraformaldehyde (9.01 g, 0.10 mol), compound IV (14.64 g, 0.12 mol), acetic acid (1.20 g, 0.02 mol), and 80 mL of benzene into the three-neck flask. Stir to dissolve, heat to reflux, remove the generated water from the reaction system with a water separator, stop heating when the water in the water separator no longer increases, and slowly cool down to room temperature. Add 100 mL of chloroform, shake vigorously and let stand, collect the organic phase, dry with anhydrous sodium sulfate, filter to remove the desiccant, and concentrate under reduced pressure to obtain a white solid powder with a yield of 81.3% and an HPLC purity of 99.44%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com