Oral pharmaceutical composition with plant alkaloid for treatment of dependencies
A composition and alkaloid technology, which is applied in the direction of drug combination, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve the problem of providing uniformity and solubility of alkaloids, and the difficulty of providing uniform content of alkaloids Sexuality, concealing the risk of overdose and other issues, to achieve the effect of improving product efficacy, reducing the risk of side effects, and reducing anxiety and fear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0030] Example No / composition 1 2 3 4 5 6 7 8 9 Cytisine 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 Galantamine HBr - - - - - - - - - Pseudine HCl - - - - - - - - - Lobetaine HCl - - - - - - - - - L-Carnitine - - - - 0.3 0.2 - - - Tryptophan - - - - - 25.0 30.0 - - Cellulose Powder 5.0 5.0 5.0 5.0 92.2 59.8 60.0 84.5 61.5 calcium sulfate dihydrate 92.5 90.0 90.0 87.5 5.0 5.0 5.0 8.0 35.0 Magnesium stearate 0.5 0.5 3.0 3.0 0.5 0.5 3.0 3.0 1.0 Anhydrous colloidal silica 0.5 3.0 0.5 3.0 0.5 3.0 0.5 3.0 1.0 total weight 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 film coating 2.0 3.0 - 4.0 5.0 - 2.0 - 3.0
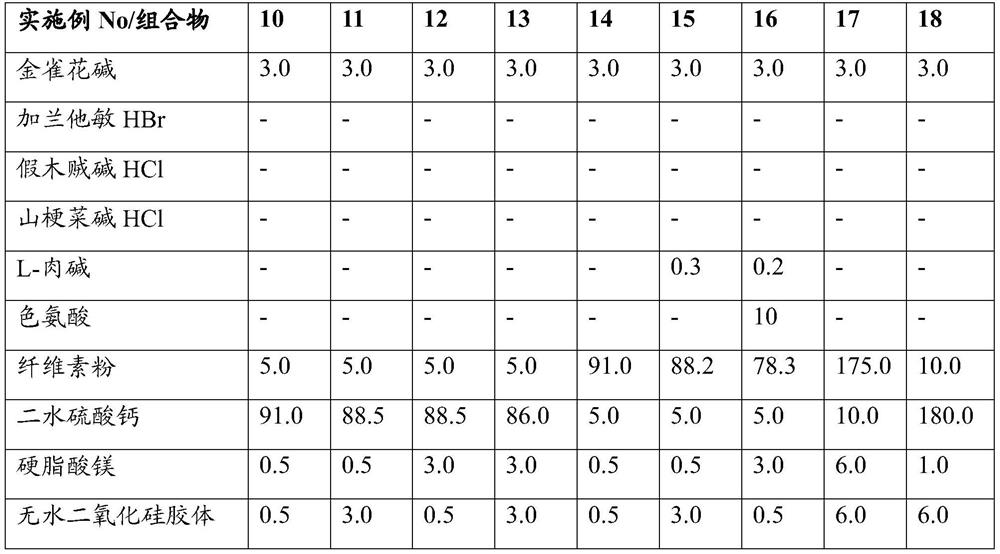
Embodiment 10-18
[0032]
[0033]
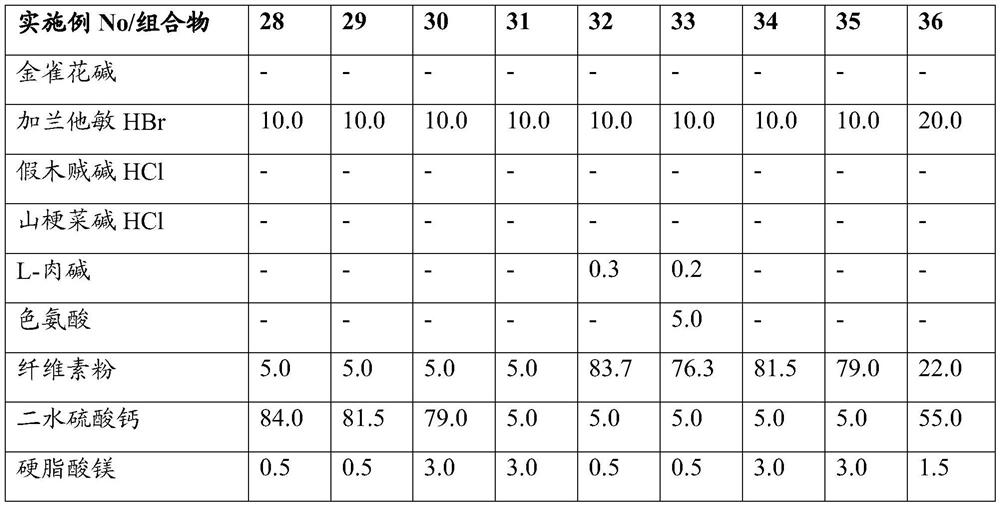
Embodiment 19-27
[0035] Example No / composition 19 20 21 22 23 24 25 26 27 Cytisine - - - - - - - - - Galantamine HBr 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 Pseudine HCl - - - - - - - - - Lobetaine HCl - - - - - - - - - L-Carnitine - - - - 0.3 0.2 - - - Tryptophan - - - - - 10.0 - - - Cellulose Powder 5.0 5.0 5.0 5.0 88.7 76.3 86.5 84.0 47.0 calcium sulfate dihydrate 89.0 86.5 86.5 84.0 5.0 5.0 5.0 5.0 45.0 Magnesium stearate 0.5 0.5 3.0 3.0 0.5 0.5 3.0 3.0 1.5 Anhydrous colloidal silica 0.5 3.0 0.5 3.0 0.5 3.0 0.5 3.0 1.5 total weight 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 film coating - 3.0 4.0 - 5.0 2.0 - 4.0 -
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com