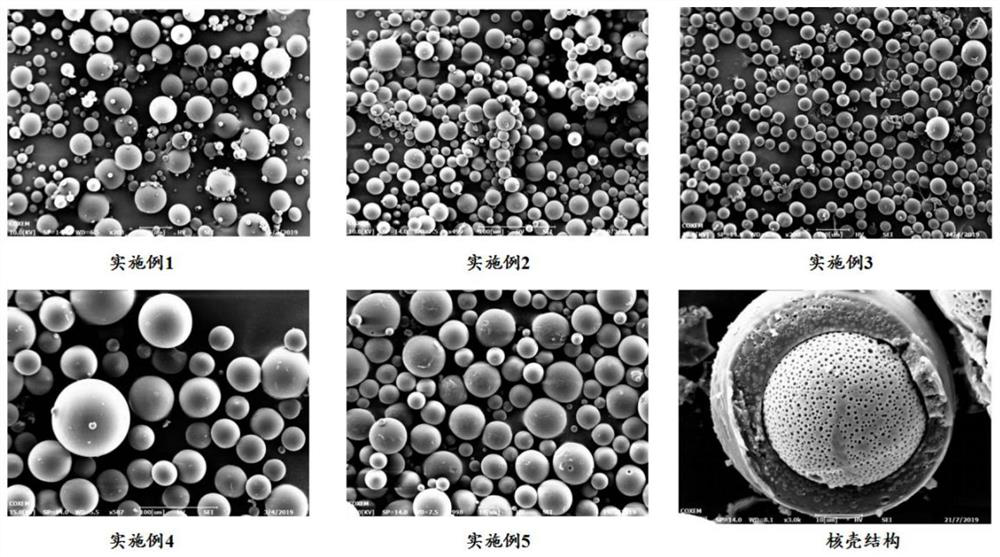
Galanthamine pamoate sustained-release microspheres for injection and preparation method thereof
A technology of galantamine pamoate and pamoic acid, which is applied in the field of drug sustained-release preparations, and can solve the problems of unfavorable process scale-up application, nozzle clogging, low drug loading and encapsulation efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
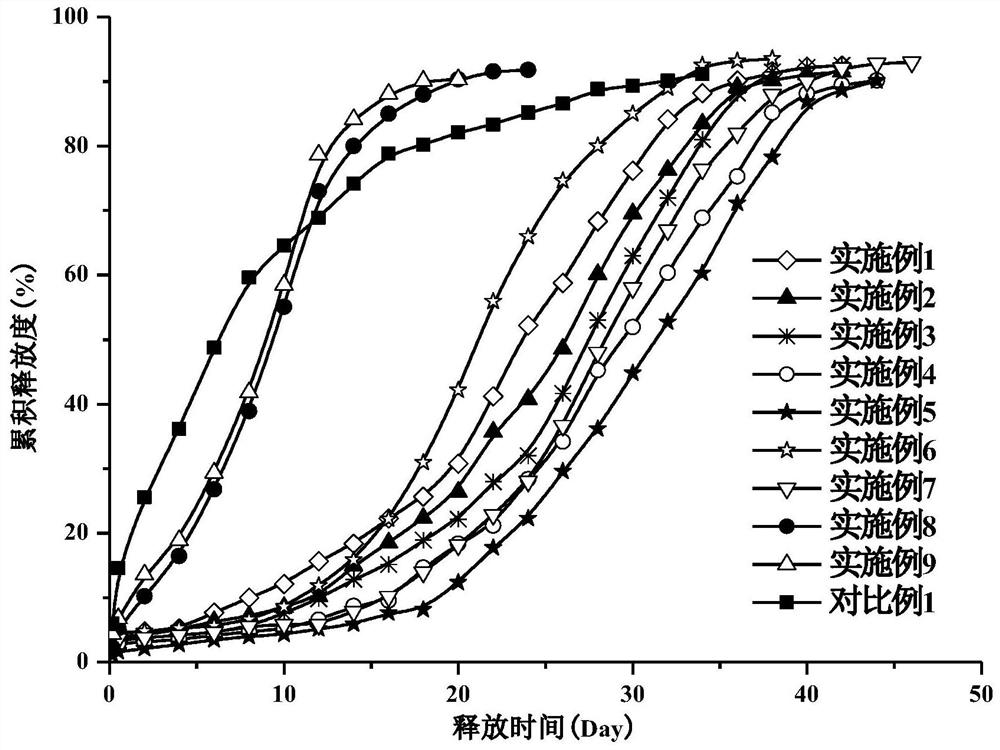
Examples
Embodiment 1~5
[0045] Examples 1-5: Preparation of microspheres with different theoretical drug loadings
[0046] Table 1. The formulations and preparation process parameters of microspheres with different drug loadings
[0047]
[0048] Preparation:
[0049] The PLGA and galanthamine pamoate were weighed respectively, stirred and dissolved in 4mL of benzyl alcohol-dichloromethane mixed solvent to prepare the continuous phase, the concentration of PLGA in the continuous phase was 300mg / mL. Weigh 6.400g of PVA and add it to 500mL water for injection (100°C), stir until dissolved, cool the PVA solution to 25°C, add water for injection to 800mL, and make 8mg / mL PVA solution to obtain the dispersed phase. Slowly add the continuous phase to the dispersed phase, and homogeneously emulsify at 3000rpm for 1min; after the emulsification is complete, turn on the mechanical stirring to evaporate the solvent, and stop stirring after 3h; filter and collect the microspheres in 800mL of poloxamer ethan...
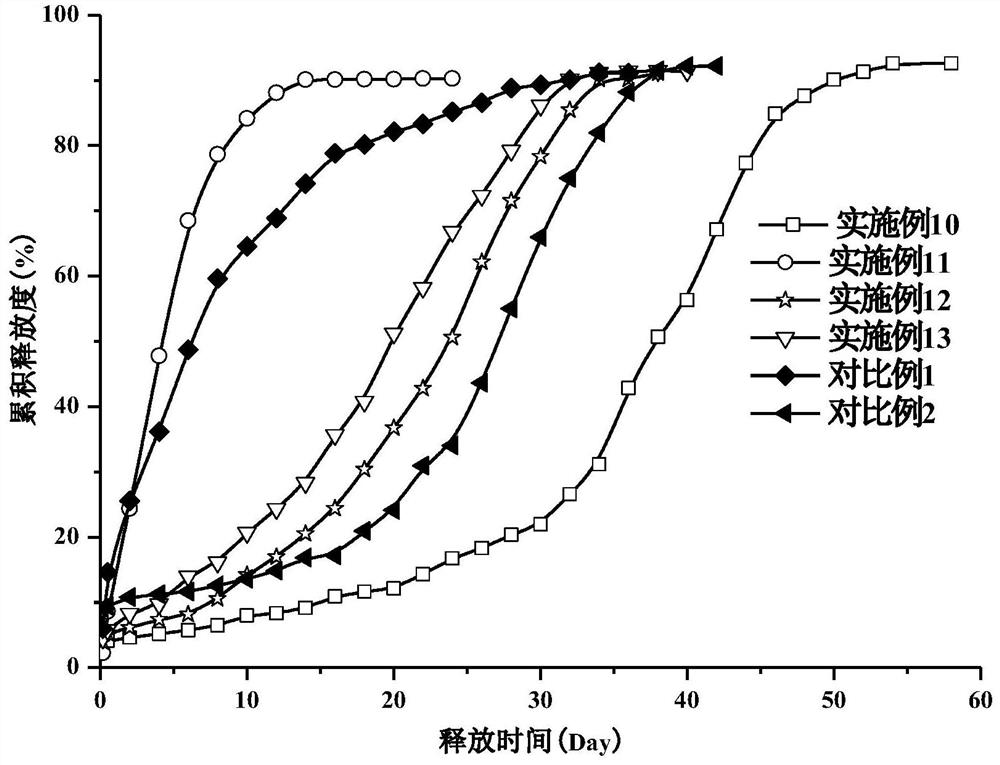
Embodiment 6
[0052] Prescription and preparation process parameters:
[0053]
[0054] Preparation:
[0055] Weigh PLGA and galanthamine pamoate respectively according to the prescription amount, stir and dissolve in 4.8mL benzyl alcohol-dichloromethane mixed solvent to prepare a continuous phase, the concentration of PLGA in the continuous phase is 250mg / mL. Weigh 8.000g of PVA and add it to 500mL water for injection (100°C), stir until dissolved, cool the PVA solution to 25°C, add water for injection to 800mL, and make a 10mg / mL PVA solution to obtain a dispersed phase. Slowly add the continuous phase to the dispersed phase, and homogeneously emulsify at 2000rpm for 1min; after the emulsification is completed, turn on the mechanical stirring to evaporate the solvent, and stop stirring after 3h; filter and collect the microspheres in 800mL of poloxamer ethanol aqueous solution, continue to stir for 1h, and The resulting suspension was filtered through a -100 mesh to +600 mesh screen t...
Embodiment 7
[0058] Prescription and preparation process parameters:
[0059]
[0060]
[0061] Preparation:
[0062] Weigh PLGA and galantamine pamoate respectively according to the prescription amount, stir and dissolve in 6mL benzyl alcohol-ethyl acetate mixed solvent to prepare a continuous phase, the concentration of PLGA in the continuous phase is 200mg / mL. Weigh 24.000g of PVA and add it to 500mL water for injection (100°C), stir until dissolved, cool the PVA solution to 25°C, add water for injection to 800mL, and make a 20mg / mL PVA solution to obtain a dispersed phase. Slowly add the continuous phase to the dispersed phase, and homogeneously emulsify at 4000rpm for 1min; after the emulsification is complete, turn on mechanical stirring to evaporate the solvent, and stop stirring after 3h; filter and collect the microspheres in 800mL polysorbate 80 ethanol aqueous solution, continue stirring for 1h, and The resulting suspension was filtered through a -100 mesh to +600 mesh sc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com