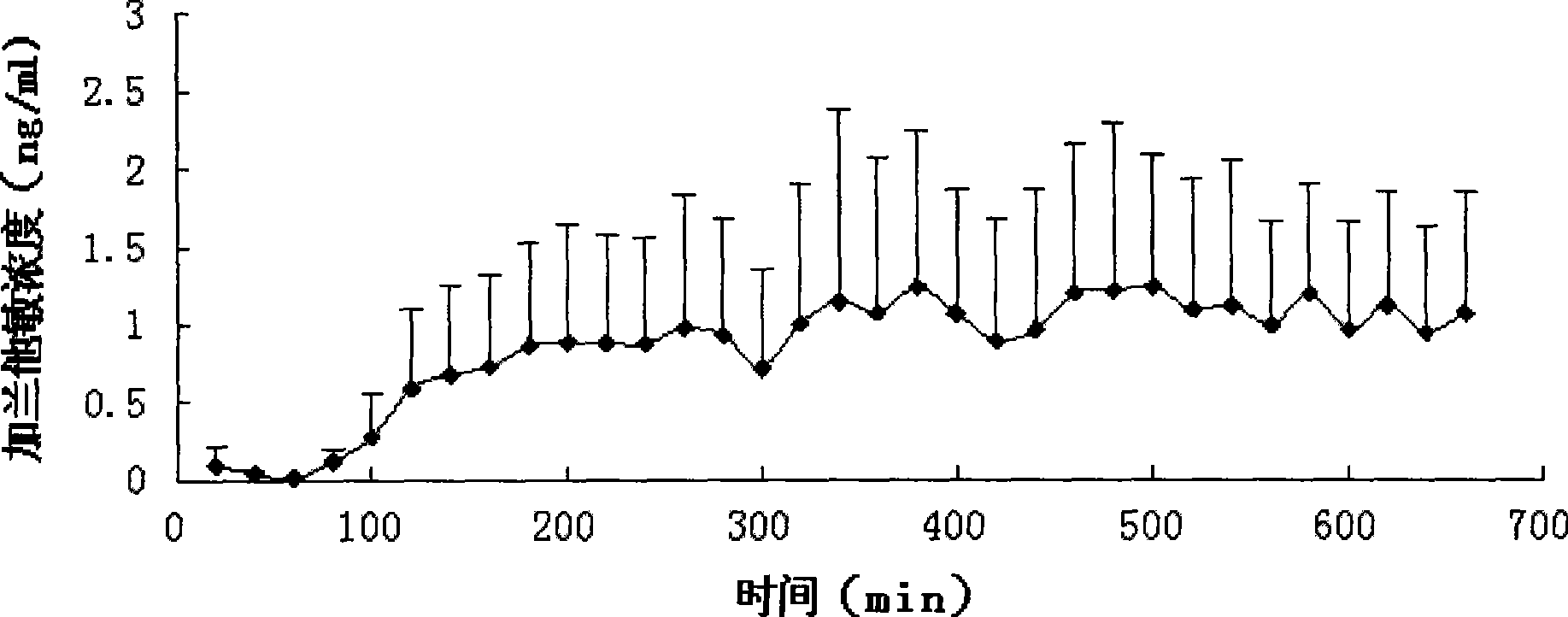
Skin paste capable of improving dementia, delusion and myasthenia due to cholinergic
A technology for skin patch and muscle weakness, applied in the field of medicine, can solve problems such as unreasonableness and fluctuation of blood drug concentration, and achieve the effect of reducing burden and improving quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1. take emulsion type acrylate pressure-sensitive adhesive as the skin patch of matrix
[0018] Formula and ratio:
[0019] Galantamine Hydrobromide 0.3g
[0020] Acrylate Emulsion Pressure Sensitive Adhesive 36.9g
[0021] Thickener 1.2g
[0022] Concentrated ammonia water 1.5ml
[0023] Purified water 6.25ml
[0024] The preparation method is:
[0025] Dissolve galantamine hydrobromide in pure water, add it to the acrylate emulsion pressure-sensitive adhesive, stir evenly while adding, add thickener, concentrated ammonia water, stir evenly, and spread on 1000cm 2 On anti-sticking paper, bake at 80°C for 30 minutes, cover the backing layer, leave at room temperature overnight, cut into 5×7cm 2 ,sealed package.
Embodiment 2
[0026] Embodiment 2. take solvent-type acrylate pressure-sensitive adhesive as the skin patch of matrix
[0027] Formula and ratio:
[0028] Galantamine Hydrobromide 0.3g
[0029] Alkaline absolute ethanol 2.0mL
[0030] Solvent Acrylic Pressure Sensitive Adhesive (38%) 46.0g
[0031] The preparation method is:
[0032] Dissolve galantamine hydrobromide in alkaline absolute ethanol (adjust absolute ethanol to pH 10 with 5mol / L NaOH solution), and add it to solvent-based acrylate pressure-sensitive adhesive (DURO-TAK 87-2677, American National Starch Company), stir evenly while adding, spread on 1000cm 2 On anti-sticking paper, bake at 80°C for 30 minutes, cover the backing layer, leave at room temperature overnight, cut into 5×7cm 2 ,sealed package.
Embodiment 3
[0033] Embodiment 3. take polyisobutylene pressure-sensitive adhesive as the skin patch of matrix
[0034] Formula and ratio:
[0035] Galantamine 3.1g
[0036] High Molecular Weight Polyisobutylene 5.3g
[0037] Low molecular weight polyisobutylene 2.8g
[0038] Liquid paraffin 5.4g
[0039] Carbopentyl resin 7.0g
[0040] 2,6-di-tert-butyl-p-cresol 14mg
[0041] Azone 1.8g
[0042] Medical gasoline 100mL
[0043] Steps:
[0044] Add the non-polar polymer high molecular weight polyisobutylene and low molecular weight polyisobutylene, plasticizer liquid paraffin and thickener carbopentyl resin to the medical gasoline according to the above ratio to completely swell, then add the drug galantamine and the transdermal accelerator Azone and antioxidant 2,6-di-tert-butyl-p-cresol, stir evenly, ultrasonically remove air bubbles, and then apply it on the anti-adhesive layer on a coating machine as usual, with a coating area of 1000cm 2, drying while coating, drying at 60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com