Method for generating multispecific antibodies from monospecific antibodies
A multimer, domain technology used in the field of generating multispecific antibodies from monospecific antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1489] Design and module composition of 2 / 3-IgG
[1490] overall evaluation
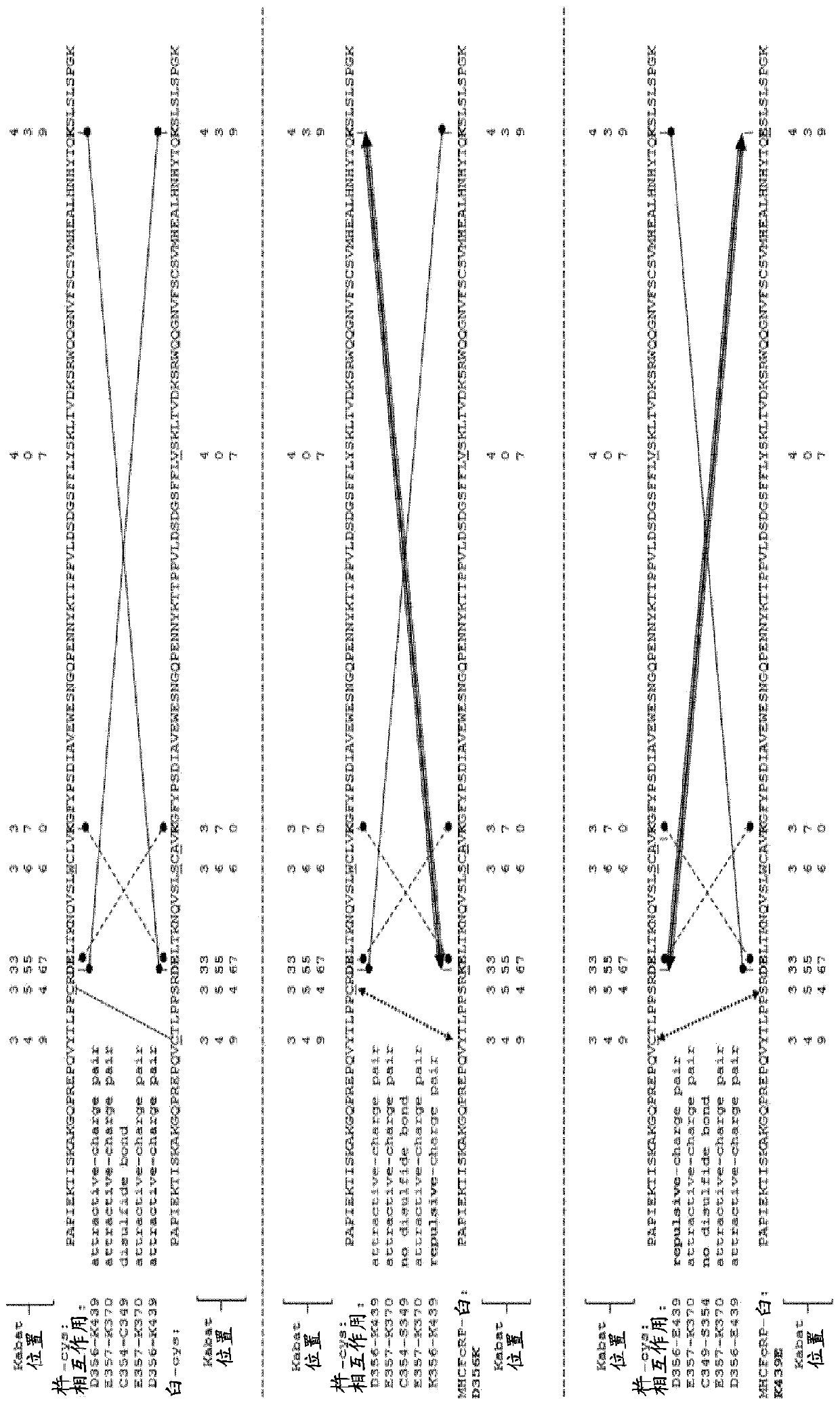
[1491] figure 1 The design and modular composition of the 2 / 3-IgG used in the method of the invention is shown. These 2 / 3-IgGs consist of three separate chains: a light chain (typically, a full-length light chain comprising the light chain variable domain and a light chain constant domain), a heavy chain (typically, Full-length heavy chain with heavy chain variable domain and all heavy chain constant domains (including hinge region), and one heavy chain Fc region polypeptide (typically, heavy chain Fc region fragment including hinge-CH2-CH3) . The light and heavy chain variable domains form a functional binding site. The heavy chain (typically, it is derived from the human IgG1 subclass) contains a knob-cys mutation or a hole-cys mutation in CH3 that enables dimerization of the knob-into-hole Fc region (in the CH3 domain of the antibody heavy chain The mutations T366W and S354C are cal...
Embodiment 2
[1497] Expression and purification of 2 / 3-IgG of the present invention
[1498] 2 / 3-IgG is achieved by co-transfection of plasmids encoding the light chain, heavy chain (with a knob mutation or a hole mutation) and matching MHC FcRP (hole or knob) into mammalian cells (e.g. HEK293) using state-of-the-art techniques Express.
[1499] In more detail, e.g., for the production of 2 / 3-IgG by transient transfection (e.g. in HEK293 cells), cDNA preparation based on the CMV-intron A promoter or a genome based on the CMV promoter was applied. Prepared expression plasmids.
[1500] In addition to the antibody expression cassette, this plasmid contains:
[1501] - origin of replication, which allows the plasmid to replicate in E. coli,
[1502] - the β-lactamase gene that confers ampicillin resistance in E. coli, and
[1503] - the dihydrofolate reductase gene from Mus musculus (Mus musculus) as a selectable marker in eukaryotic cells.
[1504] The transcription unit...
Embodiment 3
[1526] Generation of bispecific antibodies (bsAbs) via a 2 / 3-IgG-exchange reaction
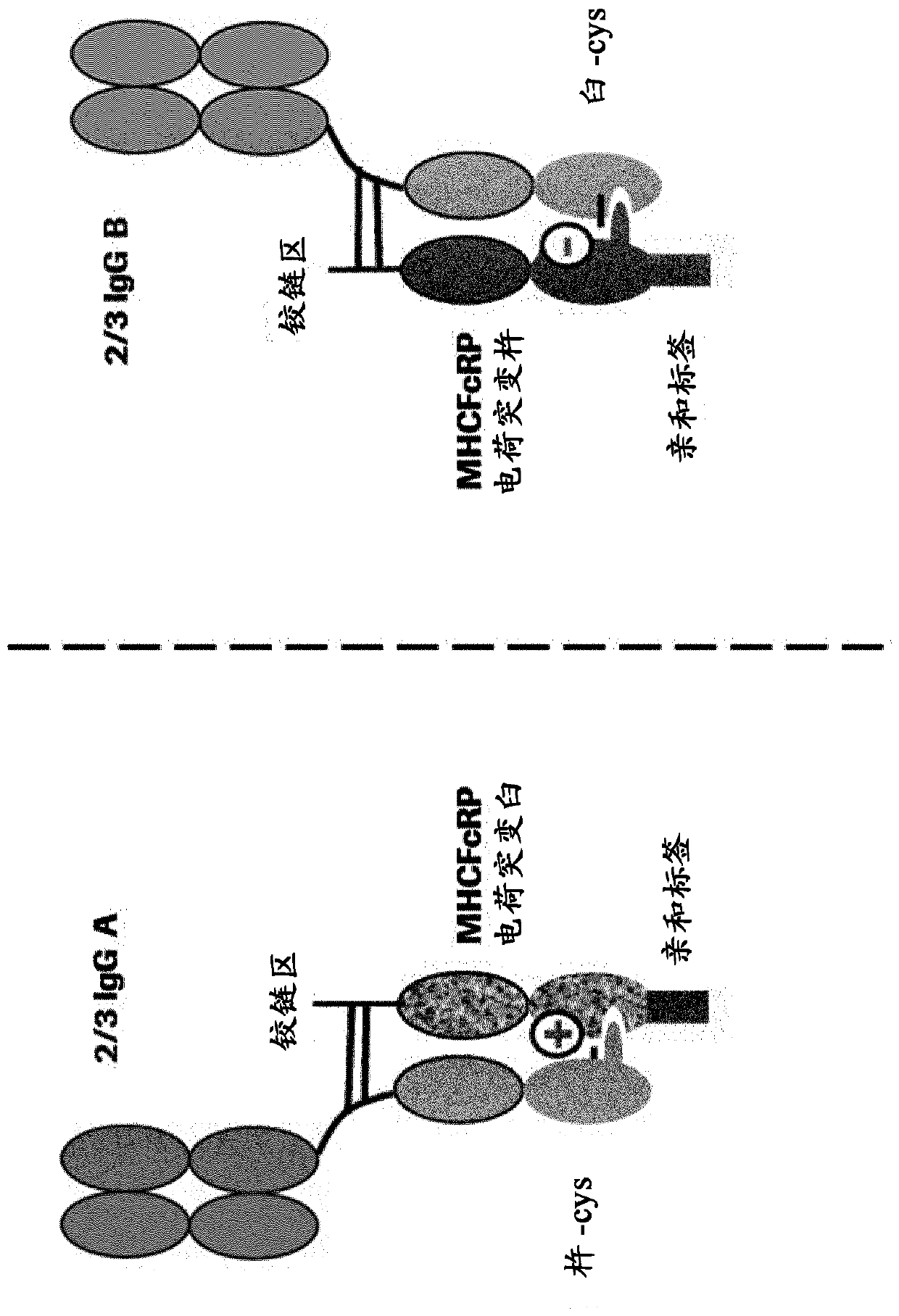
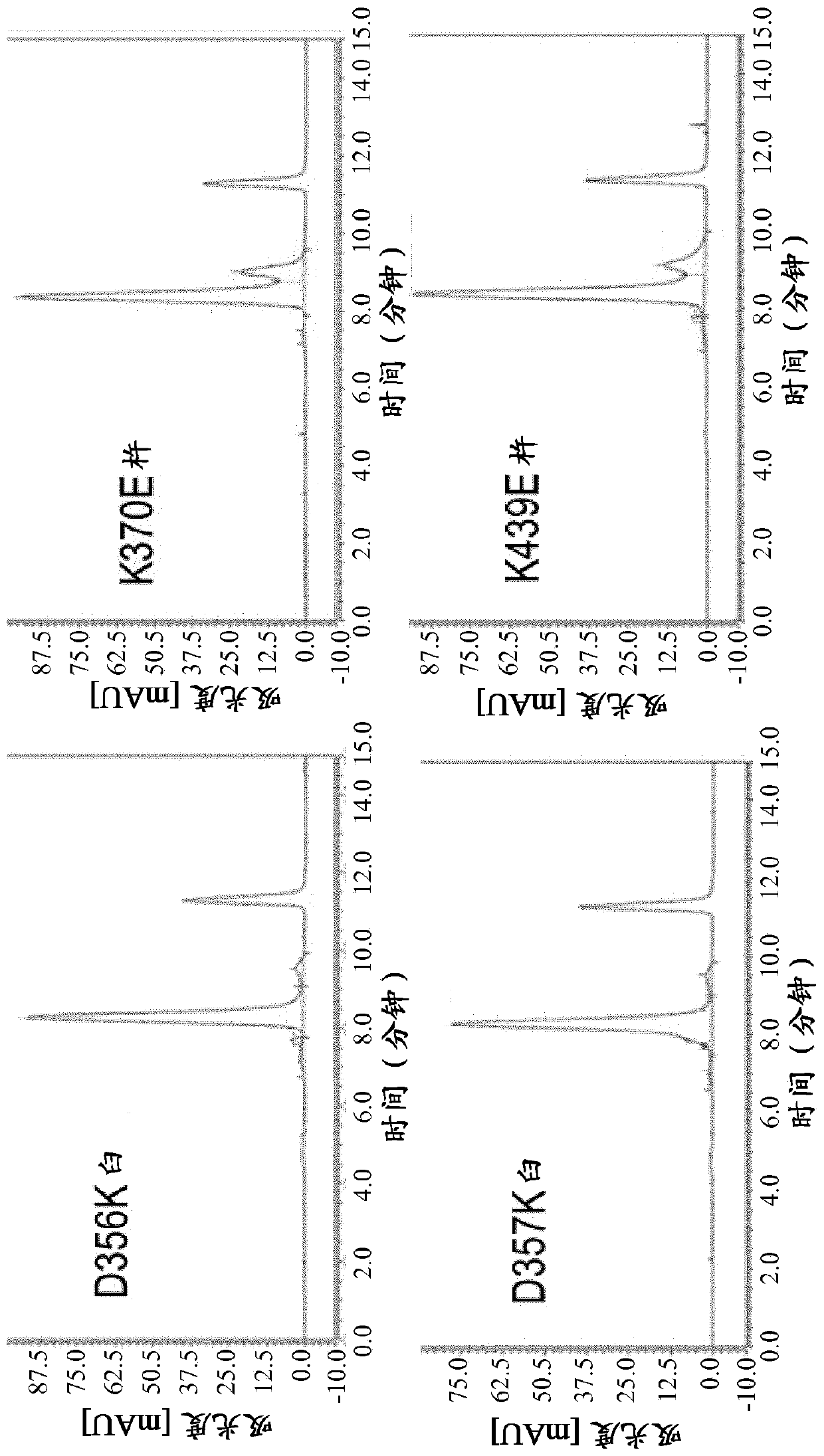
[1527] 2 / 3-IgG containing one light chain, one heavy chain and MHCFcRP has been produced as two types of KiH heterodimers: full-length heavy chain-knot::MHCFcRP-hole and full-length heavy chain-hole::MHCFcRP -Knot. Both types of 2 / 3-IgG are more or less 'flawed' because MHCFcRP lacks the additional CH3 cysteine necessary to form an interchain disulfide bond with the heavy chain, and MHCFcRP contains a mismatched full-length heavy chain The charge mutation of the counterpart. However, the modules that make up those defective heterodimers can rearrange into Figure 4 Matchingly charged bispecific heterodimers shown in . The full-length heavy chain (knob-cys) from 2 / 3-IgG A and the full-length heavy chain from 2 / 3-IgG B (hole-cys) form matching heterodimers. Compatible heterodimers are also formed when MHCFcRP (hole charge) interacts with MHCFcRP (knot charge). Thus, an exchange reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com