Combined medicine for treating HBV infection
A technology of drugs and uses, applied in the field of combined drugs for the treatment of HBV infection, can solve the problems of low conversion rate of serum HBeAg, moderate efficacy of interferon treatment, drug resistance, etc., and achieve the effects of inhibiting HBV infection, reducing the content, and having better effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] The specific implementation of the present invention will be further described below in conjunction with the examples, and the present invention is not limited to the scope of the examples.
[0017] The raw materials used in the following examples can be purchased from the market.
[0018] Experiment 1. In vitro study of the combination of lovastatin and lamivudine in HBV-infected cells
[0019] Purpose:
[0020] To evaluate the inhibitory effect of lovastatin and lamivudine drug combination on HBV infection.
[0021] method:
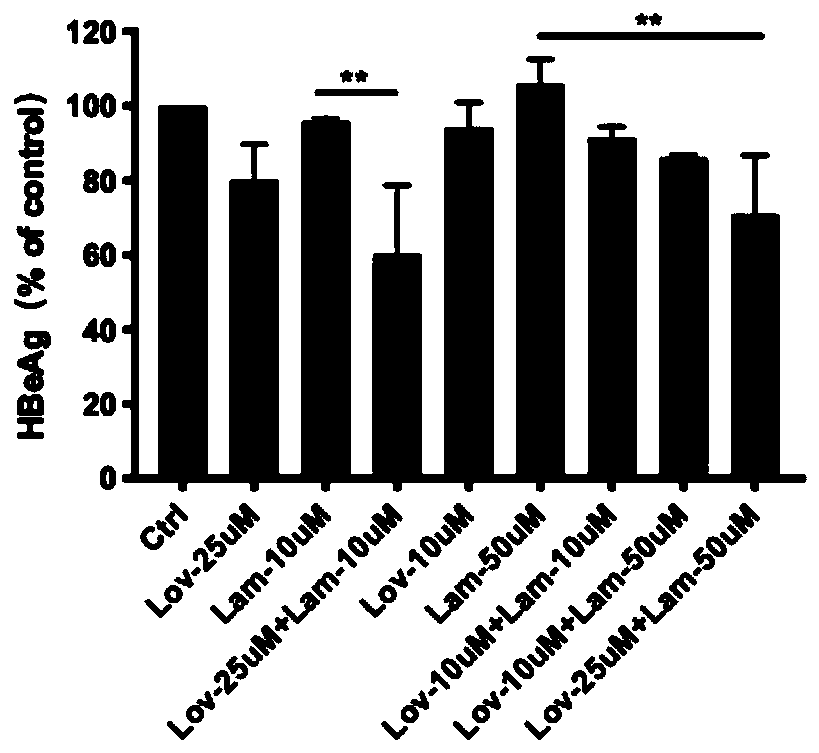
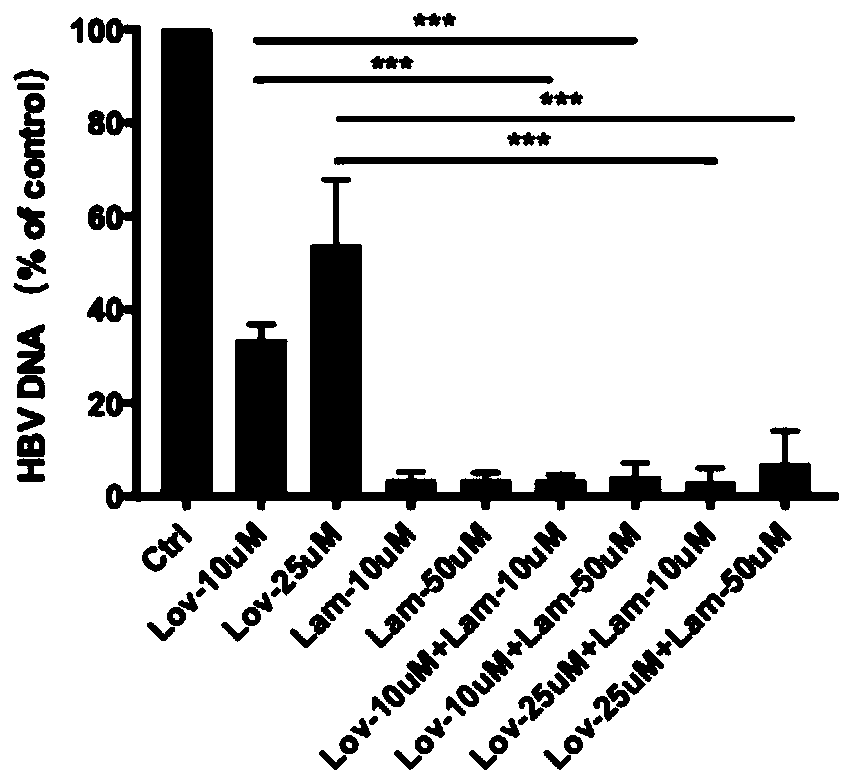
[0022] In HepG2.2.15 cells, lovastatin (10, 25 μM concentration), lamivudine (10, 50 μM concentration), and different drug concentrations of lovastatin and lamivudine were added to HepG2.15 cells, and after 6 days of culture, The supernatant was collected, the expression of HBeAg antigen was detected by ELISA, and the content of HBV DNA was detected by RT-PCR.
[0023] result:
[0024] from figure 1 It can be seen that compared with lamivud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com