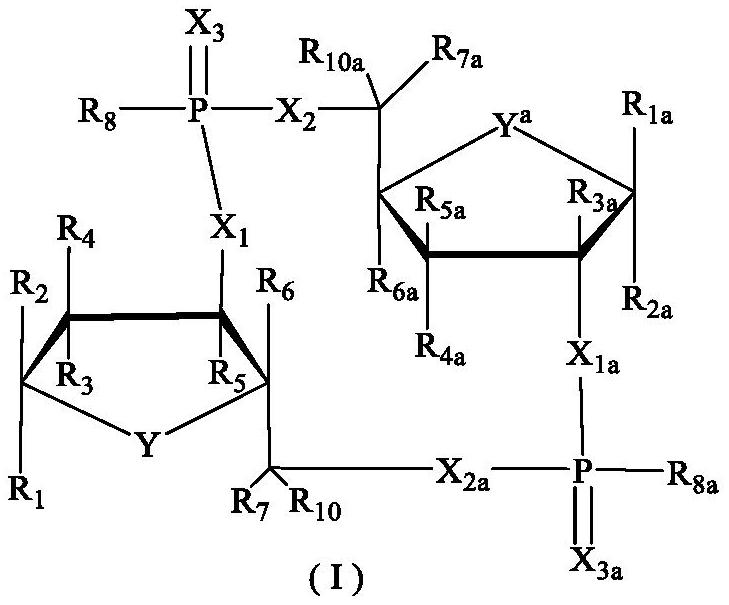
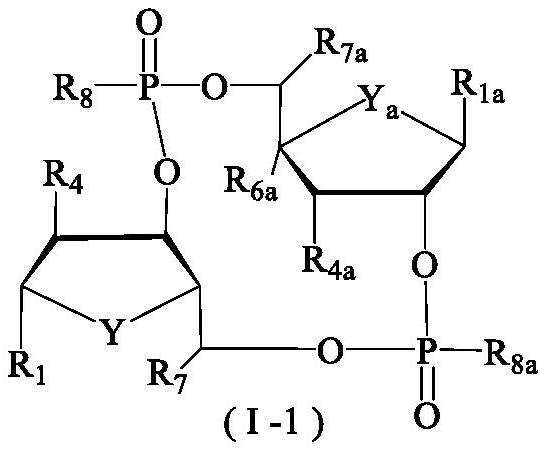
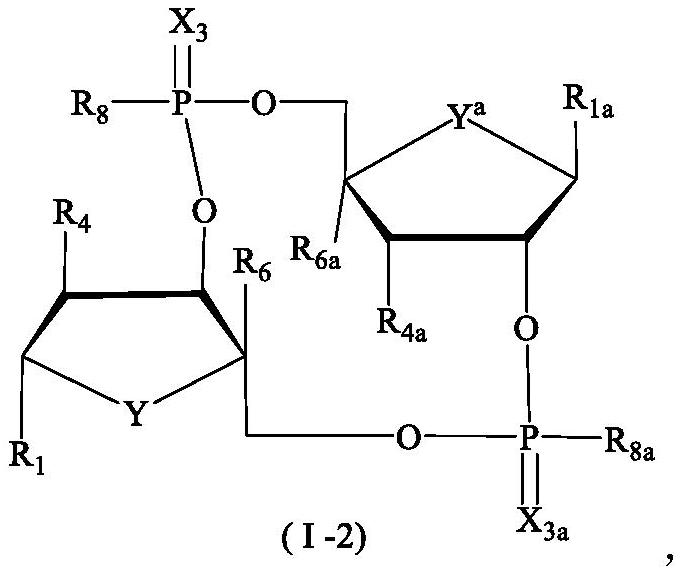
Tumor immune compounds and their applications
A compound and composition technology applied in the application field of STING agonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Embodiment 1: the preparation of compound 1A, compound 1B
[0140] Step 1: Preparation of Compound 1-2
[0141]
[0142] Under the protection of argon, the compound 4-chloro-5-fluoro-7H-pyrrolo[2,3-D]-pyrimidine (1.03g, 6.0mmol) was dissolved in acetonitrile (40mL) solution, and BSA (1.76mL, 7.2 mmol), the reaction system was stirred for 5 min and then added 1-1 (3.0 g, 6.0 mmol) and trimethylsilyl trifluoromethanesulfonate (1.32 mL, 7.2 mmol). After reacting at 25°C for 30min, the temperature was raised to 80°C for 3h. Add water (100mL) to quench the reaction, extract with ethyl acetate (100mL x 3), combine the organic phases, wash with saturated brine (100mL), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. The crude product is subjected to silica gel column chromatography Purification (petroleum ether / ethyl acetate (v / v)=5 / 1) gave compound 1-2.
[0143] MS(ESI)m / z(M+H) + =616.1
[0144] 1 H NMR (400MHz, CDCl 3...
Embodiment 2
[0223] Embodiment 2: the preparation of compound 2A, 2B, 2C, 2D
[0224] Step 1: Preparation of compound 2-3
[0225]
[0226] Compound 2-2 (1g, 3.91mmol) was dissolved in acetonitrile (20mL) solution, BSA (0.8mL, 7.1mmol) was added, and the reaction system was stirred for 5min, followed by adding 2-1 (2.0g, 5.88mmol) and trifluoroform Trimethylsilyl sulfonate (0.6 mL, 7.1 mmol). After reacting at 25°C for 5min, the temperature was raised to 80°C for 3h. Add water (50mL) to quench the reaction, extract with ethyl acetate (50mL x3), combine the organic phases, wash with saturated brine (50mL), dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and purify the crude product by silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=1 / 1), to obtain compound 2-3.
[0227] MS(ESI)m / z(M+H) + =536.2
[0228] 1 H NMR (400MHz, DMSO-d 6 )δ8.57(s,1H),8.08–8.04(m,3H),7.70(dd,J=11.4,18.8Hz,2H),7.58(t,J=7.7Hz,3H),7.51(t,J =7.8...
Embodiment 3
[0292] Embodiment 3: the preparation of compound 3A, 3B
[0293] Step 1: Preparation of compound 3-2
[0294]
[0295] Compound 3-1 (7.5g, 48.65mmol) was dissolved in methanol (2400mL), benzophenone (1.45g, 7.93mmol) was added, the reaction mixture was bubbled with argon for 1.5h, and then protected with argon, at 25°C Under these conditions, the reaction was stirred for 24 hours while irradiating with a mercury lamp. The reaction solution was concentrated under reduced pressure, and the crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=3 / 2) to obtain compound 3-2.
[0296] 1 H NMR (400MHz, CDCl 3 )δ4.73(d, J=5.4Hz, 1H), 4.31(d, J=5.4Hz, 1H), 3.94-3.83(m, 1H), 3.76-3.63(m, 1H), 2.81-2.74(m ,1H),2.61-2.47(m,1H),2.20-2.13(m,1H),1.44(s,3H),1.36(s,3H).
[0297] Step 2: Preparation of compound 3-3
[0298]
[0299] Under nitrogen protection, compound 3-2 (3.3g, 17.72mmol) was dissolved in pyridine (50mL), tert-butyldimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com