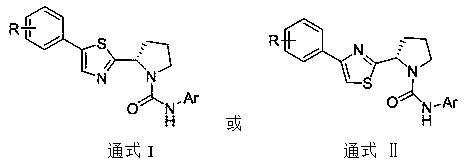
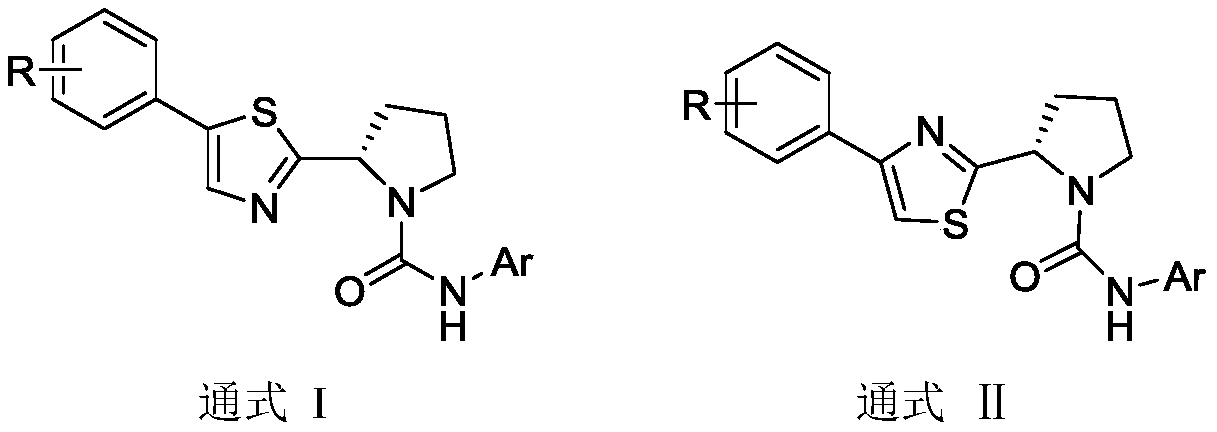
(S)-4/5-phenyl-2-(pyrrolidin-2-yl)thiazole TRPV1 antagonists as well as preparation and application thereof
A technology of phenylthiazole and pyrrolidine, which is applied in the field of preparing analgesic drugs to treat pain, and can solve problems such as gastrointestinal side effects and hematopoietic system adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Preparation of (S)-N-(2-bromophenyl)-2-(5-phenylthiazol-2-yl)pyrrolidine-1-carboxamide (1)
[0074]
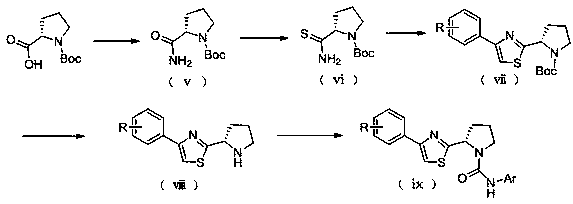
[0075] (a) Preparation of (S)-tert-butyl-2-(2-oxo-2-phenylethylcarbamoyl)pyrrolidine-1-carboxylate
[0076] Accurately weigh 1.88g (8.74mmol) of Boc-L-proline into a 100mL eggplant-shaped bottle, dissolve with 30mL dichloromethane, 1.18g (8.74mmol) of 1-hydroxybenzotriazole, 1-(3- Add 1.68g (8.74mmol) of dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) into the reaction flask in turn, stir at room temperature, and after half an hour of reaction, add 2-aminoacetophenone salt Acetate 1.00g (5.80mmol) and triethylamine 2mL, continue to react at room temperature for 18 hours, after the reaction, the reaction solution is washed with 10% citric acid solution (30mL × 3) and saturated saline solution (30mL × 3) successively , dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated to obtain (S)-tert-butyl-2-(2-oxo-2-phenyl...
Embodiment 2
[0084] Example 2: Preparation of (S)-N-(3-chlorophenyl)-2-(5-phenylthiazol-2-yl)pyrrolidine-1-carboxamide (2)
[0085]
[0086] During the preparation process, 2-bromoaniline in Example 1 was replaced with 3-chloro-aniline, and other references were made to the preparation method in Example 1 to obtain compound 2 as a brownish-yellow solid with a yield of 35%. The experimental data are as follows:
[0087] C 20 h 18 ClN 3 OS, 35% yield, dark brown solid, m.p = 140.9-1403.9°C; 1 H NMR (CDCl 3 ,400MHz): δppm 7.90(s,1H,Ar-H),7.56(t,1H,J=4.0Hz,Ar-H),7.53(d,2H,J=8.0Hz,Ar-H),7.40( t,2H,J=12.0Hz,Ar-H),7.32-7.28(m,2H,Ar-H),7.17(t,1H,J=16.0Hz,Ar-H),6.97(d,1H,J =8.0Hz, Ar-H), 5.40(dd, 1H, J=12.0, 4.0Hz, pyrrolidine-H), 3.79(t, 1H, J=8.0Hz, CH 2 ),3.68(q,1H,J=8.0Hz,CH 2 ),2.48-2.37(m,2H,CH 2 ),2.32-2.10(m,2H,CH 2 ); HRMS m / z: [M+H] + 384.0859 (calcd. 384.0931).
Embodiment 3
[0088] Example 3: Preparation of (S)-N-(4-fluorophenyl)-2-(5-phenylthiazol-2-yl)pyrrolidine-1-carboxamide (3)
[0089]
[0090] During the preparation process, 2-bromoaniline in Example 1 was replaced with 4-fluoro-aniline, and other references were made to the preparation method in Example 1 to obtain compound 3 as a brownish-yellow solid with a yield of 17.4%. The experimental data are as follows:
[0091] C 20 h 18 FN 3 OS, 17.4% yield, brown yellow solid, m.p = 127.7-128.7°C; 1 H NMR (CDCl 3 ,300MHz): δppm 7.88(s,1H,Ar-H),7.50(d,2H,J=6.0Hz,Ar-H),7.41-7.33(m,5H,Ar-H),6.95(t,2H , J=9.0Hz, Ar-H), 5.27(dd, 1H, J=12.0, 3.0Hz, pyrrolidine-H), 3.70-3.65(m, 2H, CH 2 ),2.54-2.36(m,2H,CH 2 ),2.30-2.06(m,2H,CH 2 ); HRMS m / z: [M+H] + 368.1155 (calcd. 368.1226).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com