Method for reducing content of residual alkali on surface of positive electrode material and application thereof
A cathode material, surface residue technology, applied in chemical instruments and methods, battery electrodes, nickel compounds, etc., can solve the problems of lithium-ion battery gas production, shortened cycle shelf life, and potential safety hazards, and achieves improved electrochemical performance. performance, improving the capacity fade problem, solving the effect of the hazard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
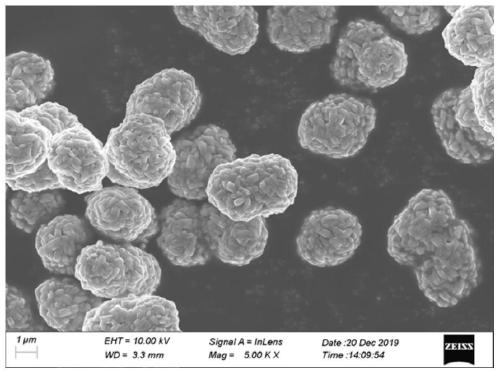
Image
Examples
Embodiment 1
[0057] Weigh 1mol of Ni 0.835 co 0.1074 mn 0.0575 (OH) 2 and 1.02mol of LiOH·H 2 O was placed in a mortar, thoroughly ground and mixed evenly, then placed in a box-type atmosphere furnace at 740°C and oxygen atmosphere for high-temperature sintering for 10 hours, and the material was taken out for ultra-centrifugal grinding and sieving to obtain the positive electrode matrix material; weigh 1mol positive electrode matrix material, 0.01mol Mn(CH 3 COO) 2 , 0.02mol LiOH·H 2 O, placed in a mortar for thorough grinding and mixing, and then placed in a box-type atmosphere furnace for high-temperature sintering at 700°C and oxygen atmosphere for 6h to obtain the final desired positive electrode material.
Embodiment 2
[0059] Weigh 1mol of Ni 0.835 co 0.1074 mn 0.0575 (OH) 2 and 1.02mol of LiOH·H 2 O was placed in a mortar, thoroughly ground and mixed evenly, then placed in a box-type atmosphere furnace at 740°C and oxygen atmosphere for high-temperature sintering for 10 hours, and the material was taken out for ultra-centrifugal grinding and sieving to obtain the positive electrode matrix material; weigh 1mol positive electrode matrix material, 0.03mol Mn(CH 3 COO) 2 , 0.06mol LiOH·H 2 O, placed in a mortar for thorough grinding and mixing, and then placed in a box-type atmosphere furnace for high-temperature sintering at 700°C and oxygen atmosphere for 6h to obtain the final desired positive electrode material.
Embodiment 3
[0061] Weigh 1mol of Ni 0.835 co 0.1074 mn 0.0575 (OH) 2 and 1.02mol of LiOH·H 2 O was placed in a mortar, thoroughly ground and mixed evenly, and then placed in a box-type atmosphere furnace at 740 ° C and oxygen atmosphere for high-temperature sintering for 10 h, and the material was taken out for ultra-centrifugal grinding and sieving; weighed 1 mol of positive electrode material, 0.03 mol Mn(CH 3 COO) 2 , 0.0598molLiOH·H 2 O, placed in a mortar for thorough grinding and mixing, and then placed in a box-type atmosphere furnace for high-temperature sintering at 700°C and oxygen atmosphere for 6h to obtain the final desired positive electrode material.
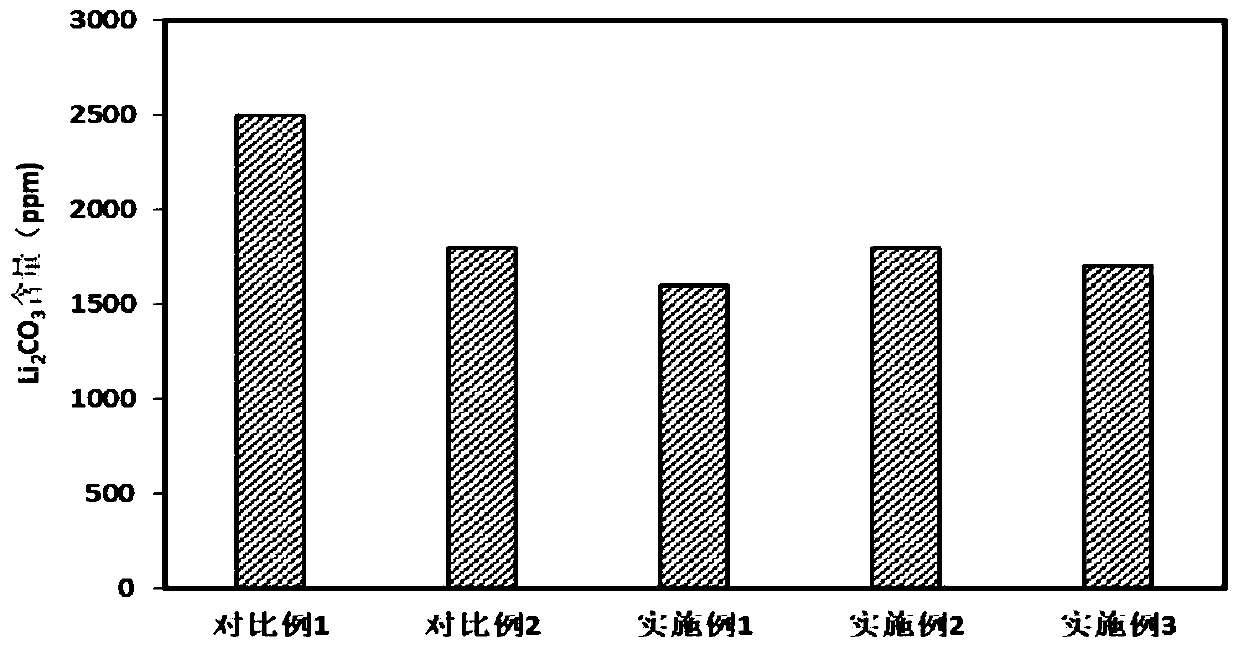
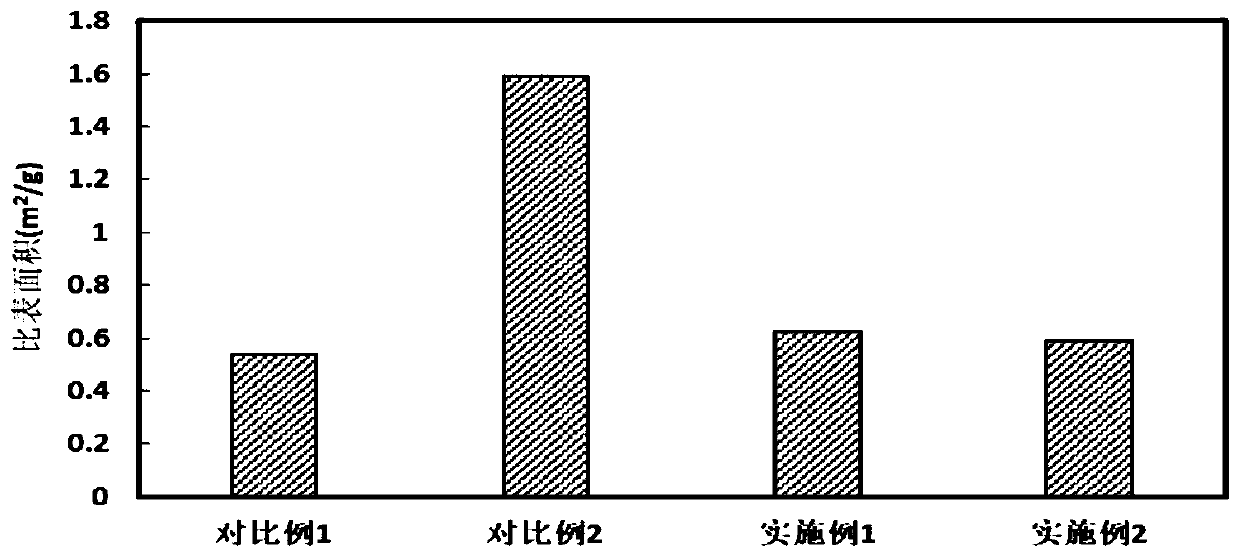
[0062] Under the same conditions, the positive electrode materials obtained in Examples 1-3 and Comparative Examples 1-2 were evaluated respectively
[0063] 1. The positive electrode materials obtained in Examples 1-3 and Comparative Examples 1-2 are tested for surface residual alkali content
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com