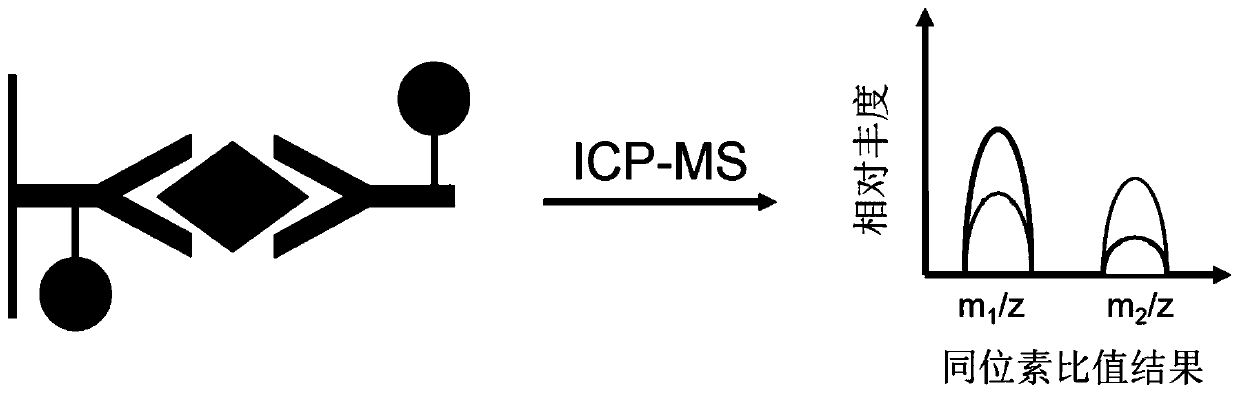
Internal standard correction-based immune precise quantitative analysis method and special kit thereof
A kit and reagent technology, applied in the direction of analyzing materials, analyzing materials by electromagnetic means, measuring devices, etc., can solve problems such as stability and consistency affecting the accuracy of quantitative results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1. Accurate immune kit based on internal standard correction and its use method
[0094] 1. Accurate immunoassay kit based on internal standard calibration
[0095] Accurate immunoassay kit based on internal standard calibration includes detection probe, internal standard probe, 96-well plate or magnetic microspheres, antigen standard, PBS buffer, washing solution (PBST solution), acid dissociation solution (nitric acid aqueous solution) .
[0096] 1. Detection probe
[0097] Use natural isotopes with relatively high isotopic abundance 153 Eu-labeled β2 microglobulin to obtain detection probes. Specific steps are as follows:
[0098] (1) DTPA (S-2-(4-Isothiocyanatobenzyl)-diethylenetriamine pentaacetic acid) compound and 153 Eu rare earth ions were dissolved in sodium acetate buffer (0.5M, pH=5.8) at a molar ratio of 1:1.2, and reacted at room temperature for 30 minutes to obtain a macrocycle-rare earth chelate.
[0099] (2) Dissolve the β2 microglobulin a...
Embodiment 2
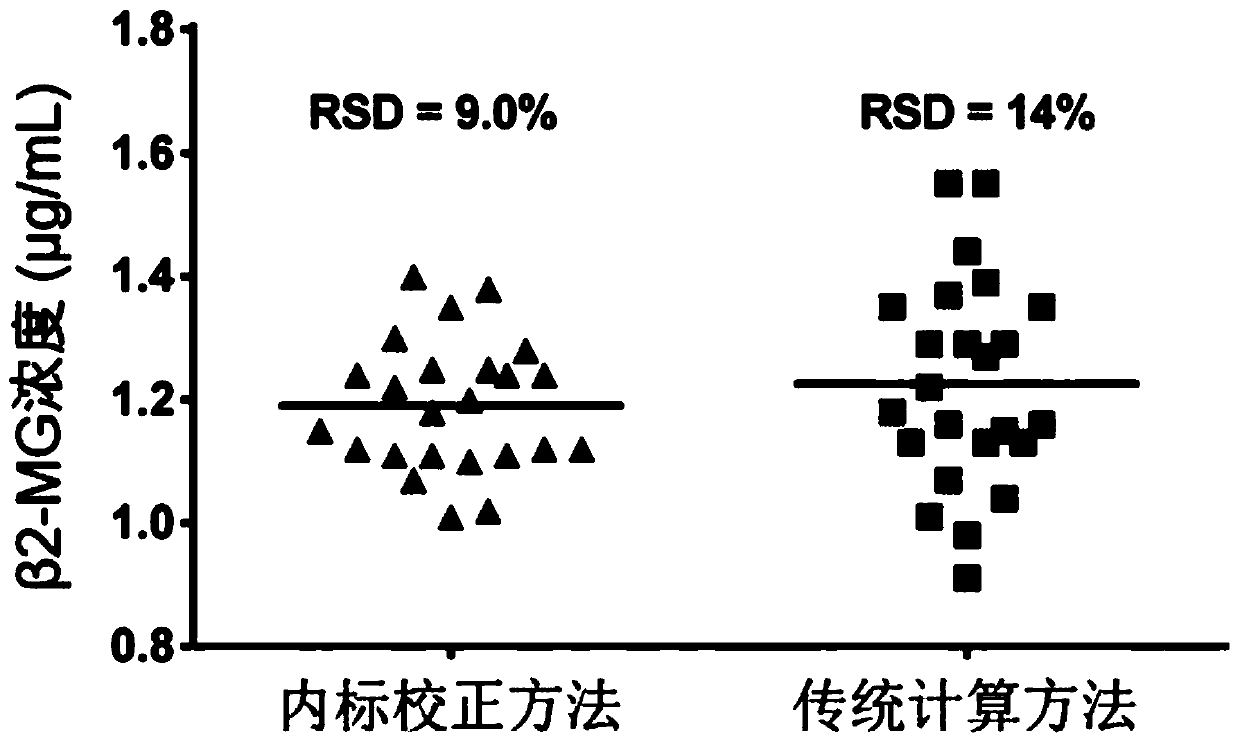
[0117] Example 2. The precise quantitative analysis method of immunity based on internal standard correction and the precision detection of traditional methods
[0118] 1. Immunoaccurate quantitative analysis method based on internal standard correction
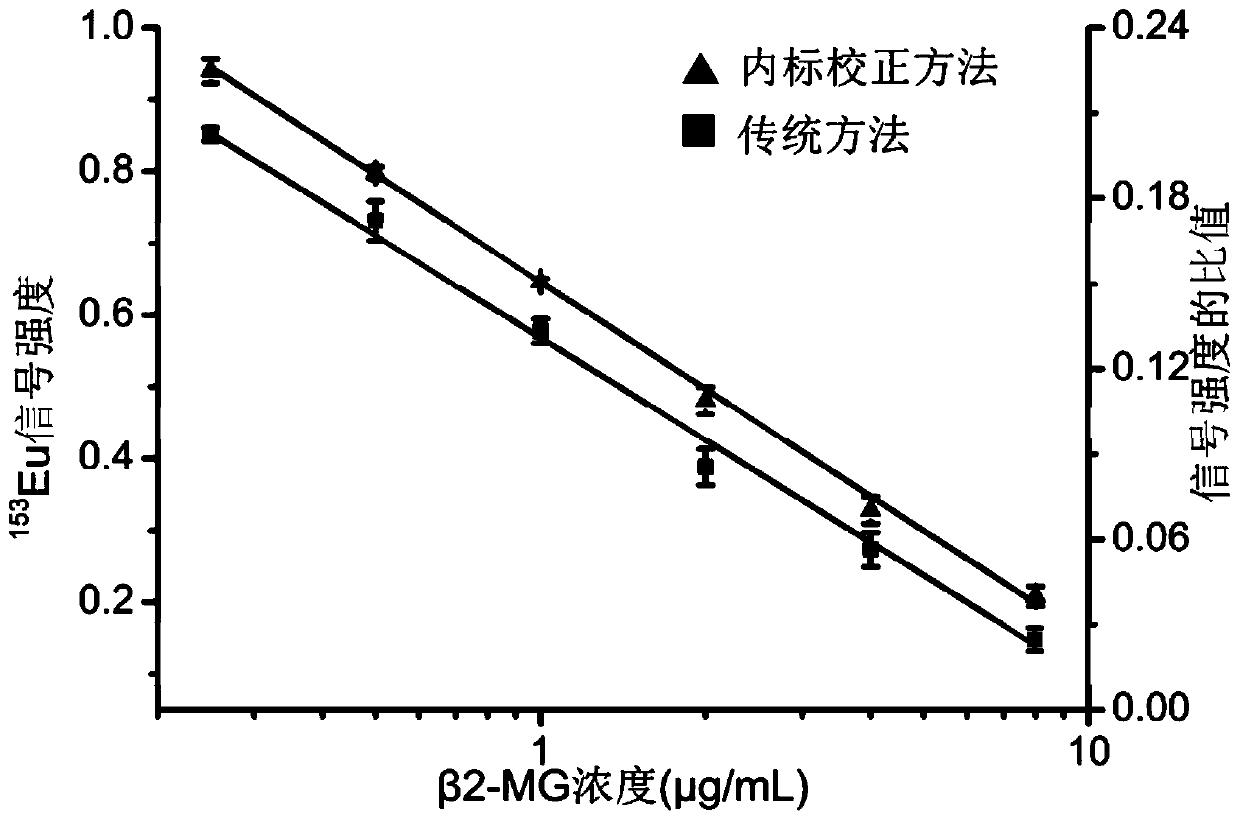
[0119] The β2-MG antigen standard substance of 1.0 μg / mL was used as the sample to be tested, and the content of β2-MG in the sample to be tested was detected using the kit and its detection method in Example 1, and the detection was repeated 6 times each time. 4 trials. according to obtained 151 Eu and 153 The signal strength of Eu and figure 2 The standard curve for the internal standard calibration method is shown, and the results for all replicate experiments calculated by the internal standard calibration method are obtained.
[0120] 2. Traditional method
[0121] The β2-MG antigen standard of 1.0 μg / mL was used as the sample to be tested, and the content of β2-MG in the sample to be tested was detected according ...
Embodiment 3
[0139] Embodiment 3, the detection of actual sample disease marker
[0140] Take 5 samples of serum from healthy subjects (informed consent of the examiners) as the samples to be tested, which are respectively recorded as sample 1, sample 2, sample 3, sample 4 and sample 5. The content of the β2-MG marker in the sample to be tested was detected by using the kit and its detection method in Example 1. According to the rare earth metal obtained 151 Eu and 153 Eu signal strength and figure 2 The concentration of β2-MG in each sample was obtained separately from the standard curve shown. The concentrations of β2-MG in sample 1, sample 2, sample 3, sample 4 and sample 5 were 1.21 μg / mL, 0.72 μg / mL, 0.48 μg / mL, 0.91 μg / mL and 1.34 μg / mL, respectively.
[0141] In order to verify the accuracy of this method, commercialized kits (β2-microglobulin detection kits, purchased from Wuxi Jiangyuan Industrial Technology Trade Corporation) were used to measure the serum levels of the abov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com