A kind of preparation method of hydroxychloroquine impurity
A technology for hydroxychloroquine and impurities is applied in the field of preparation of hydroxychloroquine impurities, and achieves the effects of reasonable design, strong operability, and simple and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A preparation method for hydroxychloroquine impurities, specifically comprising the following steps:
[0059]
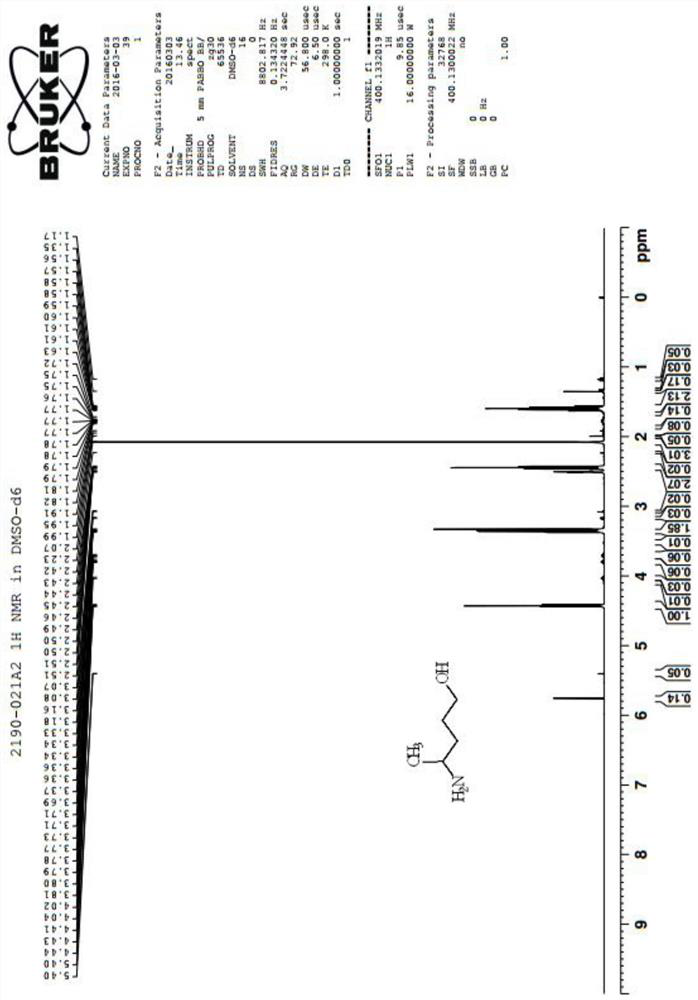
[0060] Preparation of Compound III: Mix Compound I (30.00 g) and Compound II (57.59 g), heat up to 80°C and stir for 4 hours. Cool, add methanol and dichloromethane and stir for half an hour, and the precipitated solid is suction-filtered to obtain 45.10 g of compound III as a white solid, with a yield of 94.13%. See mass spectrometry image 3 , NMR see Figure 4 .
[0061]
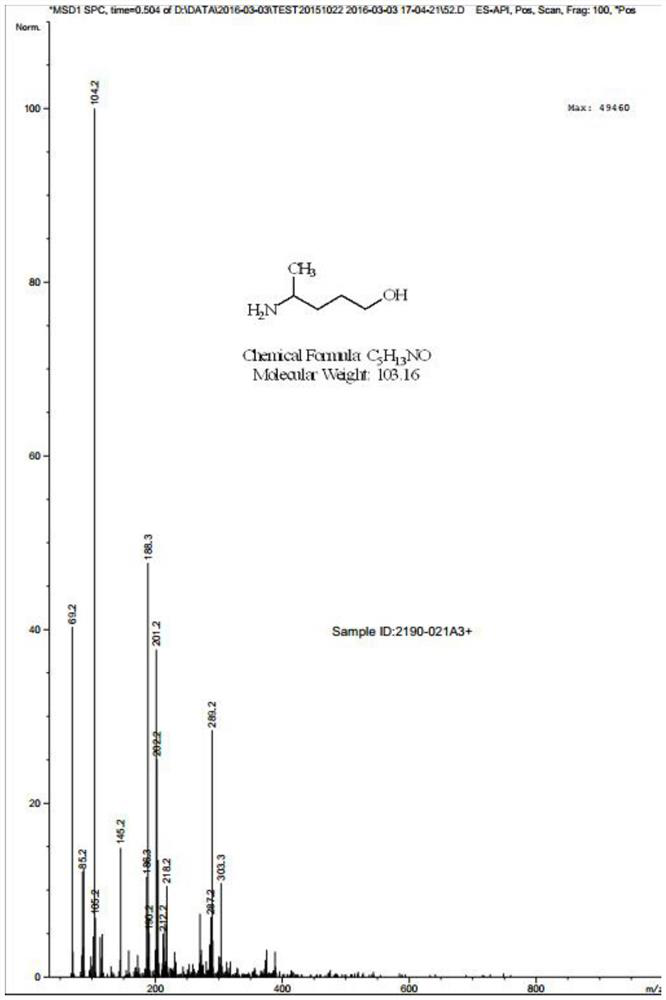
[0062] Preparation of Compound IV: Dissolve Compound III (32.00 g) in 480.0 mL of dry dichloromethane, add boron tribromide (20.40 g) and mix, and stir at 0°C for 1 hour. After quenching, adding water to adjust the base and extracting with dichloromethane to obtain 35.10 g of compound IV as a white solid with a yield of 88.63%. See mass spectrometry Figure 5 .
[0063]
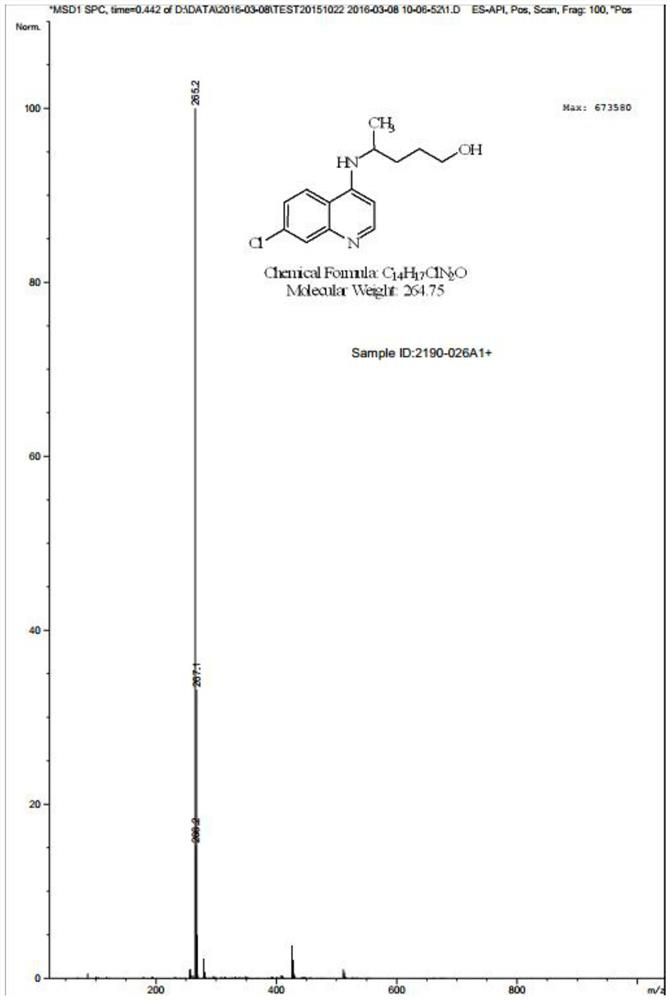
[0064] Preparation of Compound VI: Dissolve Compound IV (35.00 g) in tetrahydrofuran (350.00 mL), add 3...
Embodiment 2
[0070] A preparation method for hydroxychloroquine impurities, specifically comprising the following steps:
[0071]
[0072] Preparation of Compound III: Mix Compound I (470.00g) with Compound II (902.32g), heat up to 80°C and stir for 4 hours. Cool, add methanol and dichloromethane and stir for half an hour, and the precipitated solid is suction-filtered to obtain 1131.42 g of compound III as a white solid, with a yield of 93.80%. See mass spectrometry image 3 , NMR see Figure 4 .
[0073]
[0074] Preparation of Compound IV: Dissolve Compound III (312.00g) in 4680.0mL dry dichloromethane, add boron tribromide (198.6g), and stir at 0°C for 1 hour. After quenching, adding water to adjust the base and extracting with dichloromethane to obtain 354.20 g of compound IV as a white solid with a yield of 91.73%. See mass spectrometry Figure 5 .
[0075]
[0076] Preparation of Compound VI: Dissolve Compound IV (250.00 g) in tetrahydrofuran (2500.00 mL), add 250.0 m...
Embodiment 3
[0082] A preparation method for hydroxychloroquine impurities, specifically comprising the following steps:
[0083]
[0084] Preparation of Compound III: Compound I (10.00 g) was mixed with Compound II (7.91 g), heated to 50°C and stirred for 10 hours. After cooling, add methanol and dichloromethane and stir for half an hour, and the precipitated solid is suction-filtered to obtain 0.21 g of compound III as a white solid, with a yield of 8.18%. See mass spectrometry image 3 , NMR see Figure 4 .
[0085]
[0086] Preparation of Compound IV: Dissolve Compound III (8.00 g) in 40.0 mL of dry dichloromethane, add boron tribromide (1.00 g) and mix, and stir at 0°C for 1 hour. After quenching, adding water to adjust the base and extracting with dichloromethane to obtain 2.10 g of compound IV as a white solid with a yield of 21.21%. See mass spectrometry Figure 5 .
[0087]
[0088] Preparation of Compound VI: Dissolve Compound IV (2.50 g) in tetrahydrofuran (12.50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com