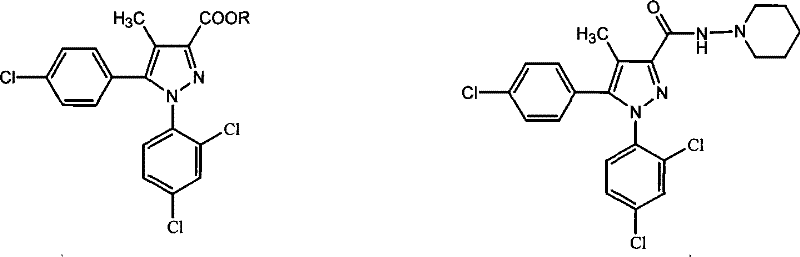
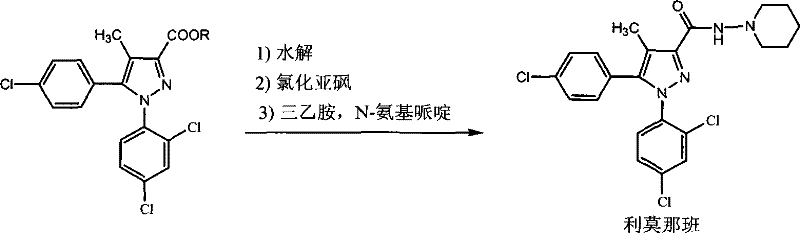
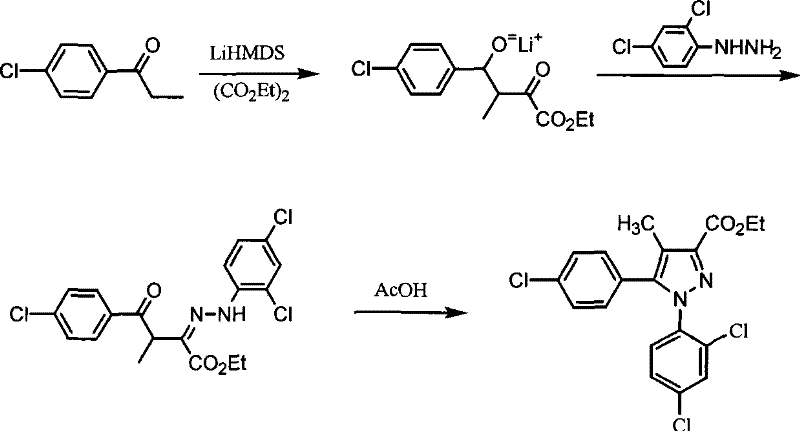
A kind of preparation method of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxylate
A technology of dichlorophenyl and methylpyrazole, applied in the field of drug synthesis, can solve the problems of high reaction cost and low yield, and achieve the effects of high yield, low synthesis cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 3.2 g (0.0755 mol) of lithium chloride to a solution composed of 6.0 g (0.0534mol) of potassium tert-butoxide and 60 ml of tert-butanol, heat and reflux for 1 hour, then distill off the tert-butanol solvent under reduced pressure . Add 40 ml of tetrahydrofuran, cool to 0-5°C and stir for 0.5 hour. Then add 5.0 g of p-chloropropiophenone (0.030 mol), stir at room temperature for 0.5 hours, then add 5.7 g of diethyl oxalate (0.0390 mol), and raise the temperature to 35-40°C for 5 hours. After the reaction, the tetrahydrofuran was distilled off to obtain the crude 4-(4-chlorophenyl)-3-methyl-2,4-dioxobutyric acid ethyl ester lithium salt as a brown-red thick substance, which was used directly In the next step reaction.
[0031] Add 50 ml of glacial acetic acid to the obtained crude 4-(4-chlorophenyl)-3-methyl-2,4-dioxobutyrate ethylenol lithium salt, cool and stir, and then add 9.6 g of 2,4-Dichlorophenylhydrazine hydrochloride (0.0450mol), and the reaction was stirred...
Embodiment 2
[0035] Add 5.2 g (0.0754 mol) of lithium nitrate to a solution composed of 6.0 g (0.0534 mol) of potassium tert-butoxide and 60 ml of tert-butanol, heat and reflux for 0.5 hours, and then distill off the tert-butanol solvent under reduced pressure. Add 40 ml of tetrahydrofuran, cool to 0-5°C and stir for 0.5 hour. Then add 5.0 g of p-chloropropiophenone (0.030 mol), stir at room temperature for 0.5 hours, then add 5.7 g of diethyl oxalate (0.0390 mol), and increase the temperature to 35-40°C for 5 hours. After the reaction, the tetrahydrofuran was distilled off to obtain the crude 4-(4-chlorophenyl)-3-methyl-2,4-dioxobutyrate ethylenol lithium salt as a brownish-red thick substance, which was used directly In the next step reaction.
[0036] Add 50 ml of glacial acetic acid to the obtained crude 4-(4-chlorophenyl)-3-methyl-2,4-dioxobutyrate ethylenol lithium salt, cool and stir, and then add 9.6 g of 2,4-Dichlorophenylhydrazine hydrochloride (0.0450mol), and the reaction was st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com