Adeno-associated viral vectors for gene therapy
A virus, amino acid technology, applied in gene therapy, virus/phage, virus, etc., can solve the problem of lack of identifiable molecules, lack of tumor-destroying molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
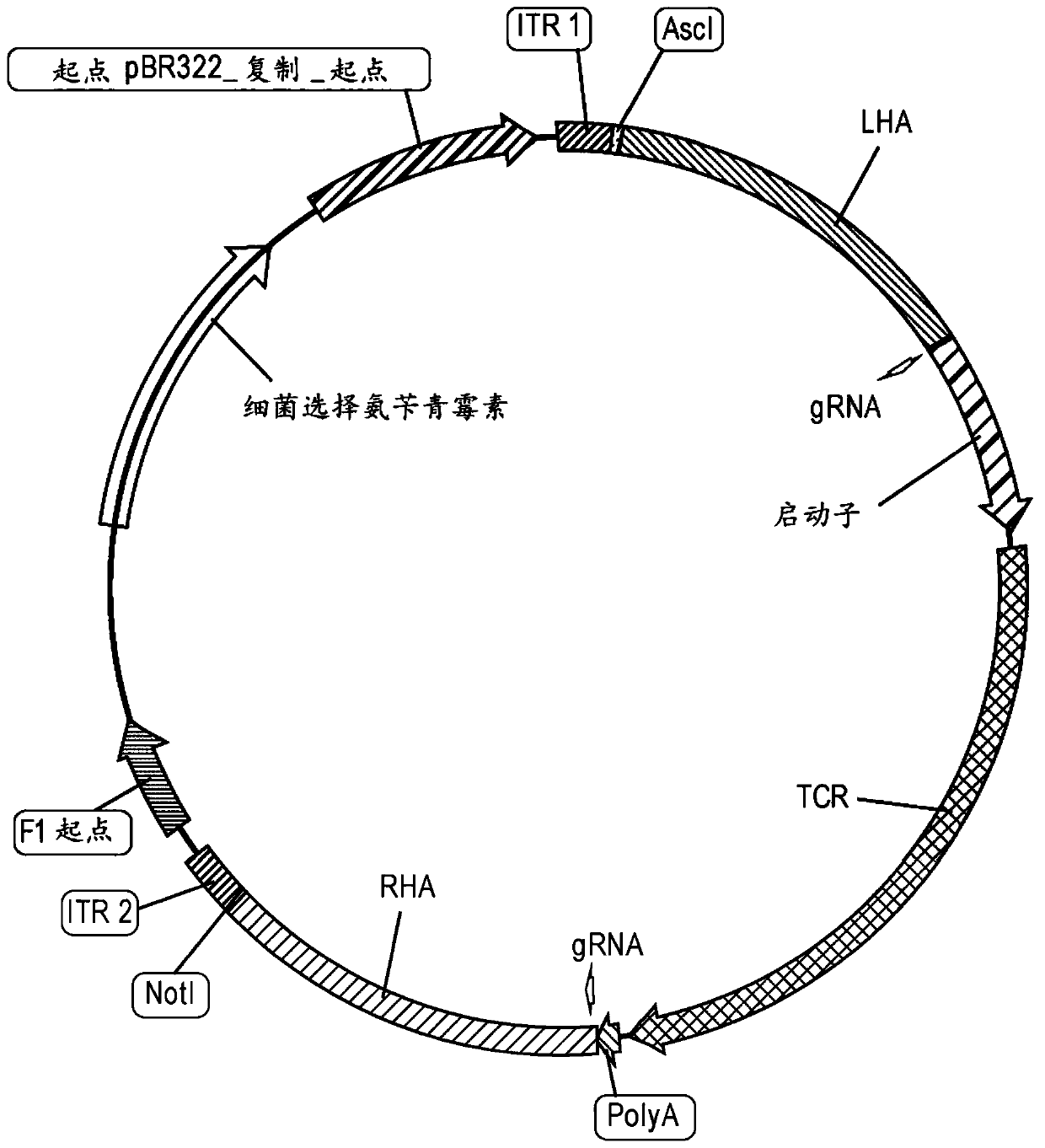
[0473] Example 1: AAV / CRISPR genome modification of human T cells Day -3: recovery and stimulation
[0474] 10% human serum was added to X-VIVO15 medium and pre-warmed at 37°C. Thaw human PBMCs in a water bath. Immediately after thawing, cells were resuspended and centrifuged at 300 g for 5-10 minutes. Cells were washed with PBS and counted by hemocytometer. Cells were then resuspended in X-VIVO15 + 10% human serum + 300 U / ml IL-2 + 5 ng / ml IL-7 and 11-15 at a concentration of one million cells / ml. Anti-CD3 and anti-CD28 Dynabeads were added and stimulation was performed at a cell:bead ratio of approximately 1 to 2. Cells were cultured for 3 days.
[0475] Day 0: Bead removal, transfection and transduction
[0476] Cells were washed with PBS and placed in DynaMag15 for approximately 1 minute. The beads were washed twice and the pelleted cells were resuspended in X-VIVO15+serum+IL-2+IL-7+IL-15 at a concentration of one million cells / ml. Cells were then incubated at 37°C ...
Embodiment 2
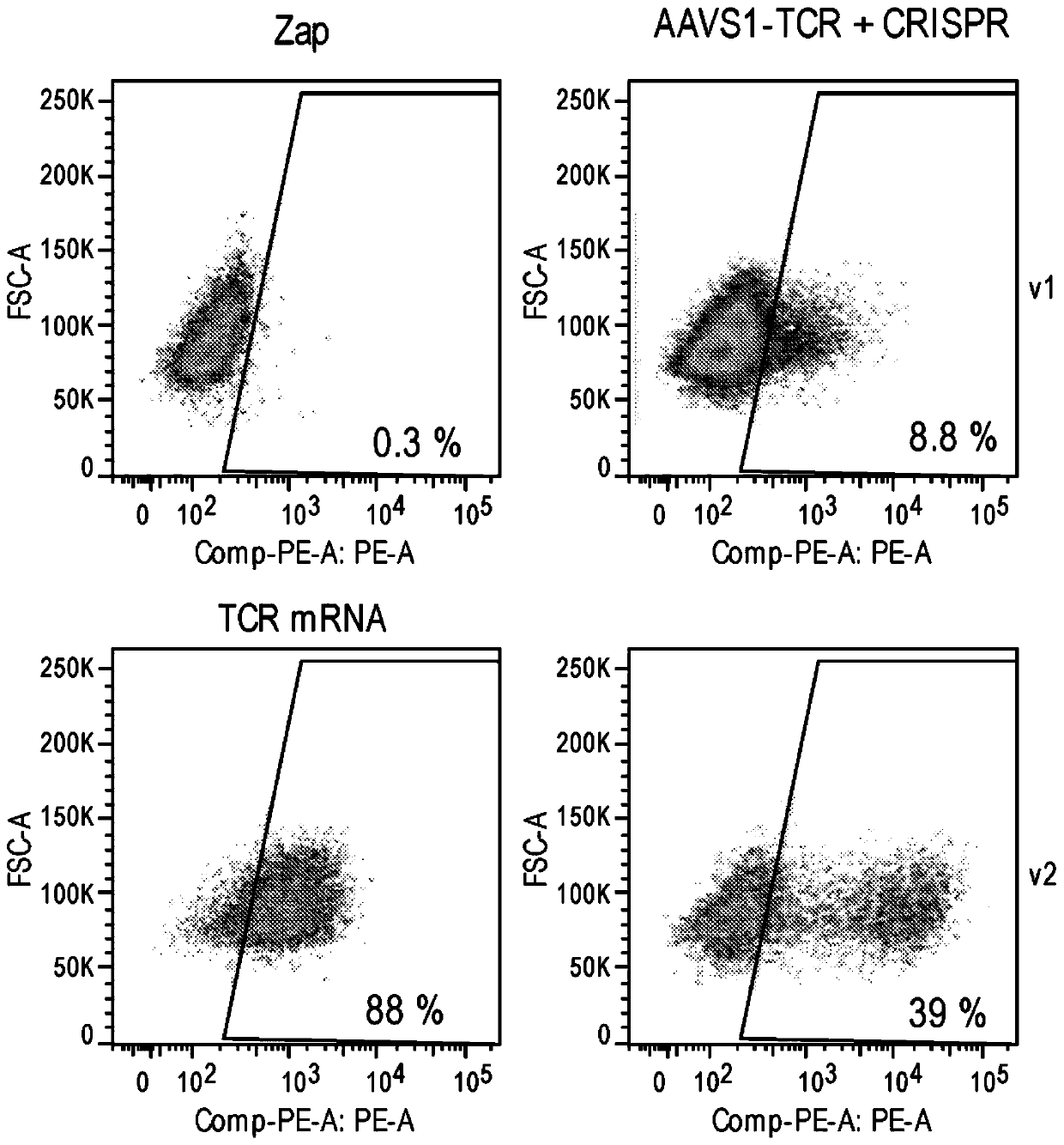
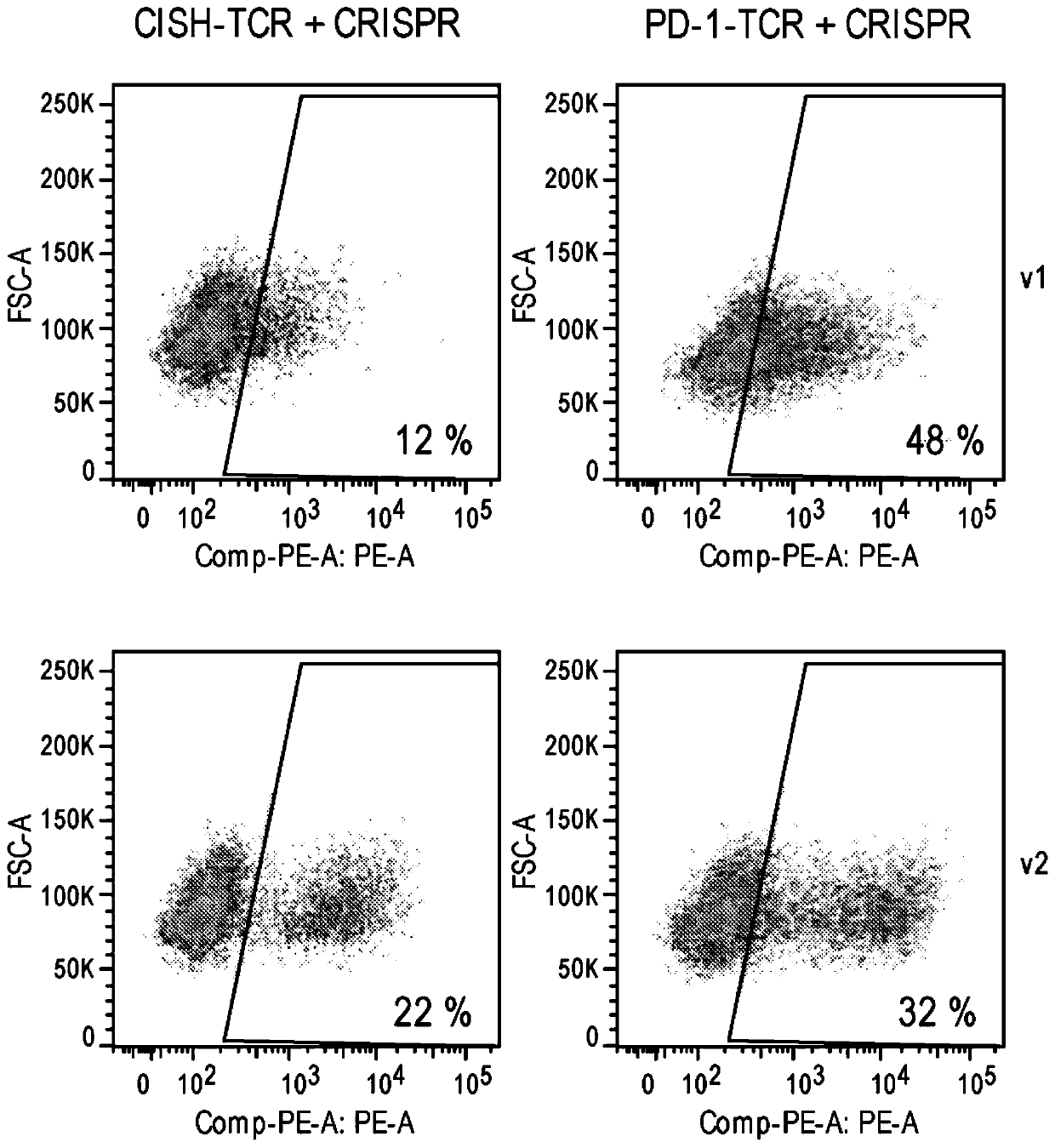
[0485] Example 2: Determination of TCR expression by flow cytometry
[0486] Cells were evaluated by flow cytometry after electroporation with CRISPR at CTLA-4, PD-1, AAVS1 and CISH and on day 3 and beyond (typically days 3, 7 and 14) after transduction with rAAV6 TCR transgene expression. Cell aliquots were collected from cultures, washed, pelleted, and resuspended in Ab (eBioscience Anti-Mouse TCR beta PE) diluted 1 / 100 in PBS + 0.5% FBS - 50-100 ul / sample. Resuspend cells in about 50 to 100 ul of Ab. Cells were incubated at 4°C for 30 minutes. The viability dye eFluor780 or SYTOX Blue viability stain was also added according to the manufacturer's instructions. After incubation, cells were washed twice in PBS and resuspended in 100 to 200 ul of PBS for analysis.
[0487] On days 3, 7 and 14, for AAV6 modified with CRISPR at CTLA-4, PD-1, AAVS1 and CISH and transduced with WT AAV6 or F129L mutated AAV6 (F129L mutated in VP1) and 2 encoding an exogenous TCR cells, their T...
Embodiment 3
[0493] Example 3: Clinical expansion of AAV-modified T cells
[0494] To generate large numbers of transduced T cells, T cell proliferation was induced using a rapid expansion protocol (REP). T cells were initially cultured with anti-CD3, anti-CD28 and IL-2 prior to use in REP and transduced as described above the day after the start of culture. Cells were incubated at 37 °C and 5% CO at 75 cm 2 cultured in flasks. Cells were counted every two days and quantified at 0.5 × 10 6 Cells / mL were suspended in fresh T cell medium containing 300 IU / mL of IL-2 and kept in culture for the rest of the time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com